Tetralogy of Fallot
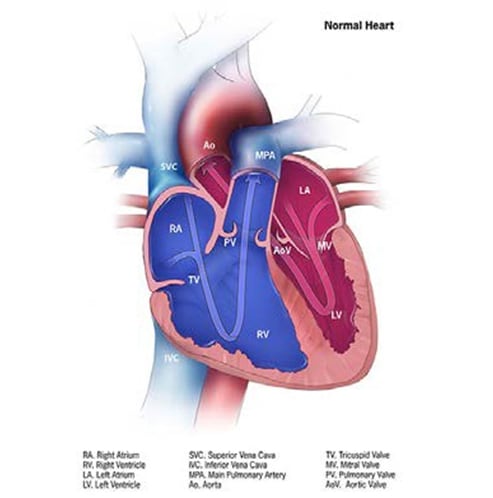
Tetralogy of Fallot is a structural heart anomaly characterized clinically by cyanosis, and anatomically by an obstructed right ventricular outflow tract associated with a ventricular septal defect (see Fig. 16); compare left panel with normal anatomy on the right).
Fig. 16. Tetralogy of Fallot
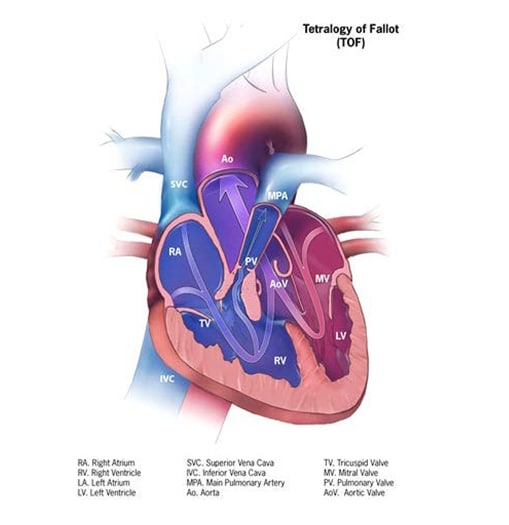
- Stenosis at or below the pulmonary valve, or pulmonary valve atresia (absent pulmonary valve can occur but is rare).
- Ventricular septal defect.
- Aorta overriding (sitting above the) ventricular septal defect.
Clinically, the classic presentation is a newborn who becomes progressively cyanotic (blue tinge most evident in the lips, nose and extremities), either constantly or intermittently, and continues to decompensate. Providing oxygen does not help, as it is not a lung problem. Non-invasive newborn screening via pulse oxymetry, though not perfect, is a helpful screening tool to detect cases that could be missed at birth by clinical examination alone. These babies require a quick diagnosis and treatment (first medical, then surgical).
Diagnosis is mainly by echocardiogram. Cases should be confirmed by a cardiologist with expertise on congenital heart disease.
Diagnosis
Prenatal. While tetralogy of Fallot can be suspected prenatally, it can be easily missed or misdiagnosed; therefore, postnatal confirmation is imperative.
Postnatal. A chest radiograph and a careful clinical examination might suggest the diagnosis; however, a definitive diagnosis is done by echocardiography. Other imaging techniques might be used but are more complex and costly (e.g. catheterization, MRI) and are used in specific, more complex cases to help with management. Newborn screening via pulse oximetry, a non-invasive approach, can detect some cases with low blood oxygenation that might escape clinical detection.
Clinical and epidemiologic notes
Tetralogy of Fallot is in fact a spectrum of diseases and might be clinically severe or mild, depending on the degree of obstruction in the right ventricular outflow tract.
- In milder cases, cyanosis might be mild or absent, and might escape detection at birth.
- Pulse oximetry, available in many nurseries, is a helpful initial tool to screen for low blood oxygenation, though not specifically for tetralogy of Fallot as there are many causes of low oxygen saturation in the newborn, including sepsis, lung disease and a variety of CHDs.
In cases of tetralogy of Fallot, always look for other birth defects and signs of genetic syndromes:
- A common genetic condition with tetralogy of Fallot (seen in about 15–20 % of cases) is deletion 22q11, a condition in which a small part of chromosome 22 is missing. This deletion leads to some CHDs and many types of birth defects, both visible externally (e.g. cleft palate, spina bifida) and internally (e.g. renal anomalies and many others) (~15–20%).
- Maternal pregestational diabetes is a modifiable risk factor for tetralogy of Fallot and other conotruncal conditions (e.g. truncus arteriosus).
Checklist for high-quality reporting
| Tetralogy of Fallot – Documentation Checklist |
Describe in detail the clinical and echocardiographic findings:
Look for and document extracardiac birth defects: In deletion 22q11, the heart anomaly can be associated with several internal and external anomalies, including cleft palate, spina bifida, vertebral anomalies, or other defects. Report whether specialty consultation(s) were done, such as whether the diagnosis was made by a paediatric cardiologist, and whether the patient was seen by a geneticist. Report any genetic testing and results (e.g. chromosomal studies, genomic microarray, etc.) |