4.5d Tetralogy of Fallot
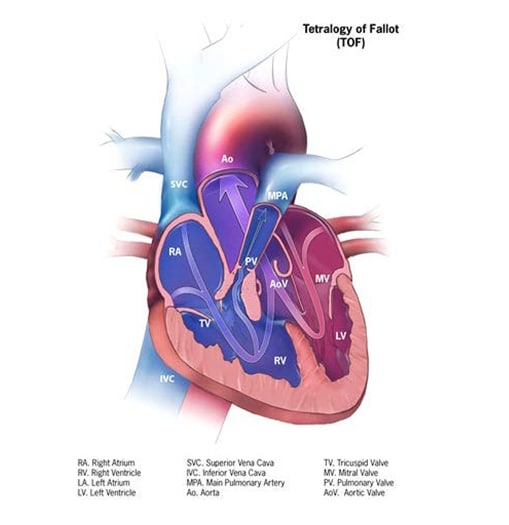
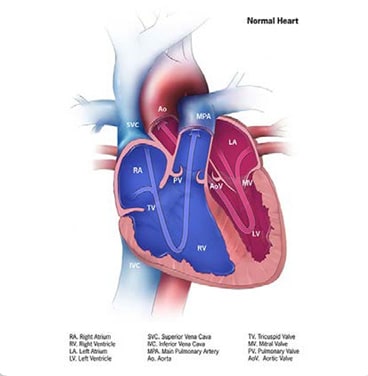
Tetralogy of Fallot is a structural heart anomaly that comprises a spectrum of disease, from severe to mild, with a common anatomic finding of right ventricular outflow tract obstruction – due to pulmonic and subpulmonic stenosis or atresia, or an absent pulmonary valve – associated with a malpositioned aorta that overrides a ventricular septal defect (see Fig. 4.17). The functional consequence is shunting/mixing of poorly oxygenated blood from the right ventricle to the aorta and thus to the systemic circulation. Depending on the degree of the shunting, which is a function of the severity of outflow tract obstruction, the child might become cyanotic, either constantly or intermittently. This form of CHD requires surgical treatment.
Fig. 4.17. Tetralogy of Fallot
Relevant ICD-10 codes
Q21.3 Tetralogy of Fallot
Q21.82 Pentalogy of Fallot (do not use, see below)
Q22.0 Pulmonary valve atresia with Q21.0 (ventricular septal defect)
Note:
- Pulmonary valve atresia with ventricular septal defect is nearly always a form of tetralogy of Fallot (a very severe form), and for this reason it is grouped with classic tetralogy of Fallot.
- Pentalogy of Fallot – which comprises tetralogy of Fallot plus atrial septal defect – is an old term seldom used now. If tetralogy of Fallot occurs with an atrial septal defect, code separately the two defects; this approach also allows the atrial septal defect to be coded using a more specific code.
Diagnosis
Prenatal. Tetralogy of Fallot can be suspected prenatally on a second trimester obstetric anatomic scan, using a combination of four-chamber and outflow tract views, and confirmed with fetal echocardiography. Detection rates are improving but diagnosis can be challenging. Postnatal confirmation is necessary for a firm diagnosis, especially to distinguish between tetralogy of Fallot with pulmonary stenosis versus with pulmonary atresia.
Postnatal. Echocardiography has largely superseded other imaging techniques. Newborn screening via pulse oximetry – which is based on the non-invasive detection of peripheral blood hypoxygenation – is effective in detecting tetralogy of Fallot as long as the condition causes the level of blood oxygenation (oxygen saturation) to go below the cut-off for newborn screening testing. For this reason, some cases of tetralogy of Fallot, especially milder cases, might be missed at newborn screening or on clinical examination before discharge from the nursery.
Clinical and epidemiologic notes
Because tetralogy of Fallot includes a spectrum of disease (depending on the severity of right ventricular obstruction), the clinical presentation and age at presentation can vary considerably, from an asymptomatic murmur in a newborn with normal or near normal oxygenation (“pink tets”) to early-onset severe cyanosis.
In some cases, there might be diagnostic uncertainty about whether the diagnosis is tetralogy of Fallot (Q21.3) or DORV (Q20.2). In this situation, the best approach is to record the primary data (e.g. reports of echocardiograms and consultations) and involve an expert clinical reviewer for final disposition.
Approximately half of cases of tetralogy of Fallot have a genetic basis, most commonly deletion 22q11 (approximately 15–20%). Tetralogy of Fallot can be found in other chromosomal conditions (e.g. the common trisomies) as well as in more than 100 single-gene conditions. Maternal pregestational diabetes is a modifiable risk factor for tetralogy of Fallot and other conotruncal conditions (e.g. truncus arteriosus).
Tetralogy of Fallot is the most common cyanotic CHD, with a frequency of approximately 1 in 2500 births.
Inclusions
Q21.3 Tetralogy of Fallot
Q22.0. Pulmonary valve atresia with Q21.0 (ventricular septal defect)
Exclusions
Q20.2 Double outlet right ventricle
Checklist for high-quality reporting
| Tetralogy of Fallot – Documentation Checklist | |
Describe in detail the clinical and echocardiographic findings:
|
Suggested data quality indicators
| Category | Suggested Practices and Quality indicators |
| Description and documentation | Review a sample of clinical descriptions for documentation of key elements:
|
| Coding |
|
| Clinical classification |
|
| Prevalence |
|