5.1 Congenital Rubella Syndrome (CRS)
Background
Rubella virus (RV) is a togavirus of the genus Rubivirus, transmitted through droplets shed from the respiratory secretions of infected persons. Teratogenic effects of infection during pregnancy can cause harm to the embryo and developing fetus. CRS is heavily underreported, as many countries lack the capacity to conduct surveillance for this condition. It is estimated that there are approximately 105 000 CRS cases worldwide each year. Countries with sustained high rates of immunization have greatly reduced or eliminated rubella and CRS.
Main clinical manifestations in the mother
The incubation period of RV infection is 14 days (range, 12–23 days). Clinical symptoms include mild illness with low-grade fever (< 39 °C), headache, conjunctivitis and rhinitis. A characteristic feature is post-auricular, occipital and posterior cervical adenopathy (swelling of the lymph nodes), which precedes a red, maculopapular rash by 5–10 days. The rash occurs in 50–80% of rubella-infected persons, begins on the face and neck, and progresses to the lower parts of the body, lasting about three days. In 70% of women, joint pain (arthralgia) also occurs.
Main clinical manifestations in the infant
If primary rubella infection occurs during pregnancy, the virus can infect the placenta and fetus, causing a constellation of specific malformations labelled CRS. The classic triad of clinical manifestations associated with CRS among surviving neonates are hearing impairment, congenital heart defects – in particular, branch pulmonary artery stenosis and patent ductus arteriosus – and eye anomalies such as cataract(s), pigmentary retinopathy (salt and pepper type), chorioretinitis or congenital glaucoma. Additional clinical signs include skin purpura (blueberry muffin skin lesions), splenomegaly (enlargement of the spleen), microcephaly (small head circumference), developmental delay, meningoencephalitis, low birth weight, radiolucent bone disease and jaundice within 24 hours after birth (Fig. 5.1). The periconception period and early pregnancy (8–10 weeks) are the most vulnerable time frames and pose the greatest risk of CRS, which is as high as 90%. CRS can result in fetal death. For infants with CRS, hearing impairment, eye symptoms and developmental delay might not be detected until later.
If maternal rubella infection is diagnosed beyond 18 weeks of gestation, the fetus might be infected but does not typically develop signs and symptoms of CRS. Infants with laboratory evidence of rubella and without any signs or symptoms of CRS are classified as having congenital rubella infection (CRI) only.
Fig. 5.1. Clinical findings in the infant
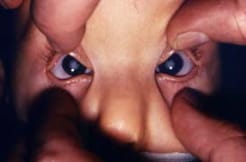
Infant with typical cloudiness of the eye lenses; that is, cataracts, in a case of CRS.
Photograph source: CDC public health image library/Dr Andre J. Lebrum
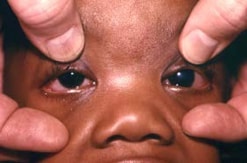
Congenital glaucoma (and cataract) in a 7-month-old infant with CRS. The left eye displays a congenital cataract; the right eye is normal. The infant was operated on on day 3 of life to correct the congenital cataract.
Photograph source: CDC public health image library/Dr Andre J. Lebrum
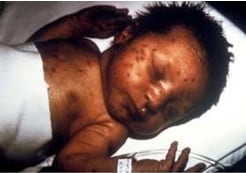
Infant with congenital rubella and “blueberry muffin” skin lesions. Lesions are sites of extramedullary hematopoiesis and can be associated with several different congenital viral infections and hematologic diseases.
Photograph source: CDC public health image library/Dr Andre J. Lebrum.
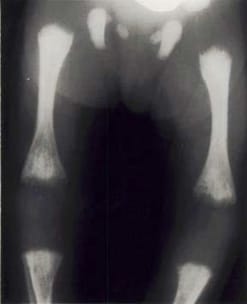
Radiolucent bone disease. X-ray of the lower limbs in a newborn with CRS. The ends of the long bones are ragged and streaky (like celery stalks) – changes due to active rubella infection.
Photograph source: Government of Canada web page (Public health/ Rubella).
Diagnosis
Laboratory:
- Rubella immunoglobulin M (IgM) antibody detected (infants < 6 months old) in serum; or
- Sustained rubella immunoglobulin G (IgG) antibody level detected in serum; present on at least two occasions between 6 and 12 months of age (in the absence of having received rubella vaccine or being exposed to rubella); or
- Rubella virus detection (nucleic acid amplification tests [NAATs]* or rubella virus isolation) from a clinical sample. The optimal sample is a throat swab; however, nasal swabs, blood, urine or cerebrospinal fluid are also acceptable.
* Nucleic acid amplification tests (NAATs) include, but are not limited to, reverse transcriptase polymerase chain reaction (RT-PCR), real-time RT-PCR, quantitative RT-PCR, and next-generation sequencing.
Infants:
In CRS and CRI cases, IgM might be negative at birth and a suspected infant should be tested again at 1 month of age. Although IgM might persist up to 12 months of age, in 50% of the cases, IgM is negative at 6 months of age, and should be complemented with IgG testing. The IgG testing should include serial testing to ensure sustained levels of IgG after 10 months (when maternal antibodies have waned) and before vaccination. Testing should also include virus detection (through NAATs or virus isolation) over several months for virus shedding until testing negative at two occasions at least one month apart. Infants with CRS or CRI have been demonstrated to shed virus up to 27 months after birth and might be the source of rubella outbreaks.
Pregnant women:
Pregnant women exposed to rubella should undergo IgM testing of serum. If IgM for rubella is detected in a pregnant woman with no history of illness or contact with a rubella illness case, further laboratory investigation to rule out a false-positive test result is warranted.
In some countries, pregnant women are routinely screened for rubella IgG antibody. If IgG antibody is negative, vaccination is recommended after delivery because the vaccine contains live virus. Rubella vaccination during pregnancy should be avoided.
Case definition
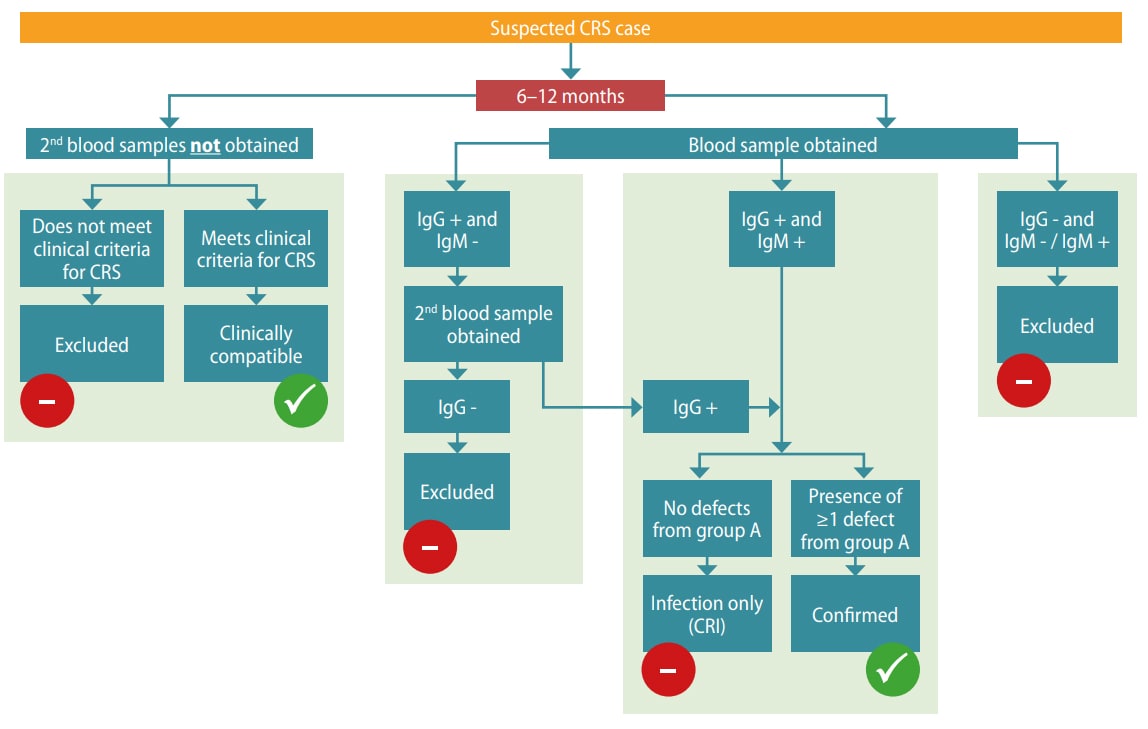
Clinical diagnosis alone is unreliable and should be verified with laboratory testing. Routine surveillance of CRS is focused on infants < 12 months of age (Table 5.1). The algorithm for CRS case confirmation in infants < 6 months and 6–12 months is presented in Fig. 5.2.
Table 5.1. CRS case definition
| Suspected CRS case |
|
| Clinically confirmed CRS case |
|
| Laboratory-confirmed CRS case | An infant who is a suspected case with one condition from group A (as above) and meets the laboratory criteria for CRS laboratory confirmation. |
| CRI | An infant who does not have group A clinical signs of CRS but who meets the laboratory criteria for CRS is classified as having congenital rubella CRI. |
a cataract, opaque or white lens; b congenital glaucoma, enlarged eyeball; c purpura, blueberry muffin skin lesions; d splenomegaly, enlarged spleen
Fig. 5.2. CRS case confirmation
CRS case confirmation in infants <6 monthsa
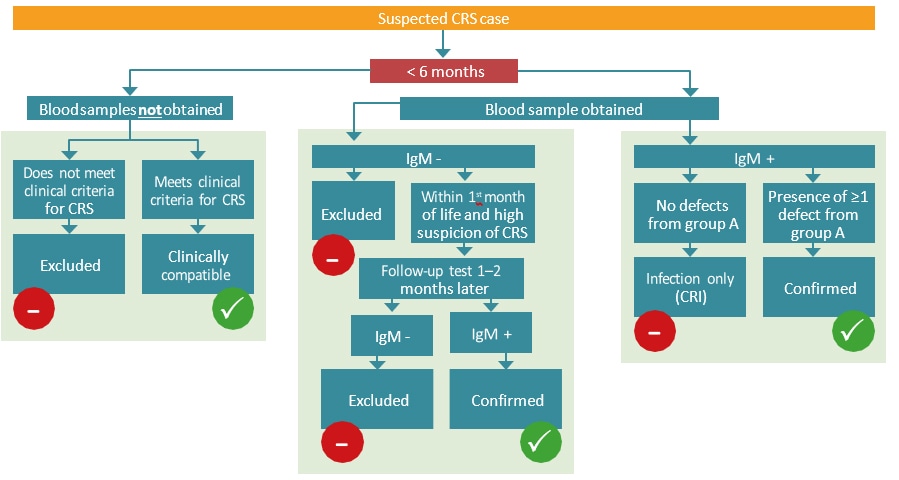
a Testing should also include virus detection (through NAAT or virus isolation) over several months for virus shedding until testing negative at two occasions at least one month apart.
CRS case confirmation in infants 6 -12 months
Relevant ICD-10 codes
P35.0 Congenital rubella syndrome (CRS)
Q02 Microcephaly
Q12.0 Congenital cataract
Q15.0 Congenital glaucoma
Q25.0 Patent ductus arteriosus
Q25.6 Stenosis of pulmonary artery
Checklist
Examine the neonate for:
- Eye: Glaucoma, cataracts, chorioretinitis or pigmentary retinopathy (salt and pepper) and the sclera for jaundice
- Skin: Jaundice that begins within 24 hours after birth and purpura
- Abdomen: Splenomegaly
- Cardiac: Murmur
- Neurological system: Developmental delay, meningoencephalitis.
Additional clinical examinations:
- Skeletal radiograph: Radiolucent bone disease
- Hearing screening test: Hearing impairment (failed hearing screening must be followed with diagnostic testing to verify hearing loss)
- Echocardiography: CHD.
Inquire about maternal medical health and pregnancy history to ascertain rubella infection and vaccination status. Lack of a history of known infection should not preclude suspicion of CRS.
Collect neonatal samples for laboratory testing (IgM and IgG).
Determine whether the case is suspected, clinically confirmed, laboratory-confirmed, CRI, or excluded.
Obtain photographs of any malformations noted
Record diagnostic information and report.