5.4 Congenital Zika Syndrome (CZS)
Background
Zika virus (ZIKV) is a flavivirus (family Flaviviridae), an RNA virus primarily transmitted by Aedes mosquitoes. These mosquitoes generally bite during the day, with peak times in the morning and early evening. These mosquitoes also transmit dengue, chikungunya and yellow fever viruses. Transplacental (vertical) and sexual transmission of the virus have been documented, as well as transmission by blood transfusion. Although Zika virus has been detected in human milk, transmission through breastfeeding has not been definitively demonstrated.
Main clinical manifestations in the mother
The risk of a pregnant woman acquiring a primary infection is the same as that of other adults. Symptoms of ZIKV infection are generally mild, non-specific and self-limited, lasting two to seven days. Symptoms vary and might include maculopapular rash, low-grade fever, conjunctivitis, muscle and joint pain, arthritis, malaise and headache. However, a large percentage of infections are asymptomatic.
The most concerning feature of ZIKV infection is maternal infection during pregnancy, which poses a risk of congenital ZIKV infection and resultant birth defects in the fetus. ZIKV infection should be suspected based upon symptoms and exposure (residence in or travel to an area with active ZIKV transmission) or sexual contact with a person who has been exposed, with confirmatory laboratory testing whenever possible.
Main clinical manifestations in the infant
Congenital ZIKV infection can lead to a spectrum of birth defects. Severe manifestations can result in a recognized pattern of birth defects known as CZS. Although many of the components of this syndrome – such as cognitive, sensory and motor disabilities – are shared by other congenital infections, there are five features that are rarely seen with other congenital syndromes or are unique to congenital ZIKV infection (see Fig. 5.5):
- severe microcephaly with partially collapsed skull and redundant scalp with rugae (extra skin folds);
- thin cerebral cortices with subcortical calcifications;
- macular scarring and focal pigmentary retinal mottling;
- congenital contractures of major joints (arthrogryposis)‡; and
- marked early hypertonia or spasticity and symptoms of extrapyramidal involvement‡.
‡ Noted in infants with structural brain anomalies only.
Since the publication of initial clinical descriptions, three additional features that appear to be unique to congenital infection with ZIKV compared with other established congenital infections include paralysis of the diaphragm, hypertensive hydrocephalus following severe microcephaly, and neurogenic bladder. The first two features appear to be low-prevalence findings. Neurogenic bladder appears to be a common finding among infants with CZS phenotype; however, numbers tested are small.
Other anomalies commonly reported with congenital ZIKV infection can be seen with congenital CMV but less so compared with other congenital infections, including cortical atrophy, corpus callosal agenesis/hypoplasia, cerebellar (or cerebellar vermis) hypoplasia, neuronal migration defects such as gyral anomalies or heterotopia, periventricular calcifications, hydrocephalus ex vacuo, glaucoma, and postnatal-onset microcephaly. Additional anomalies that are common to a number of congenital infections (including CZS) are microcephaly, hydrocephaly/ventriculomegaly/colpocephaly, calcifications of basal ganglia or unspecified regions, hearing loss, porencephaly, hydranencephaly, microphthalmia/anophthalmia, optic nerve hypoplasia, coloboma, and cataracts – these anomalies cannot reliably be used to differentiate among congenital infections.
Primary ZIKV infection during the first and early-second trimesters of pregnancy is more commonly reported in infants with adverse outcomes that are severe. Infection in the third trimester is associated with less severe defects of the brain and eyes. Milder cognitive effects such as learning disabilities have also been reported but are not yet linked to a specific trimester of exposure. Congenital ZIKV infection has been associated with other adverse birth outcomes, such as miscarriage, stillbirth and neonatal death, but studies are not conclusive.
Fig. 5.5. Clinical findings in the infant
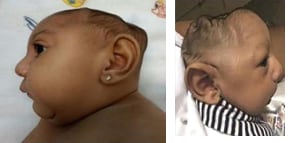
Lateral views showing collapse of the cranium and extreme reduction in height of the cranial vault.
Photograph source: Moore et al., 2017 (34); Ritter et al., 2017 (35)

Posterior scalp view showing redundant scalp with folds (rugae).
Photograph source: Moore et al., 2017 (34).
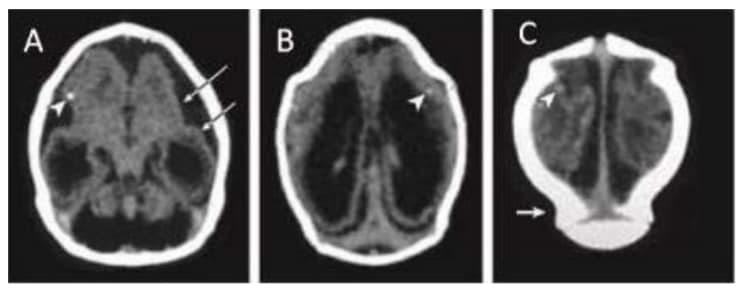
Computed tomographic (CT) scan in one infant with prenatal ZIKV exposure shows scattered punctate calcifications (A,
B and C; white arrowheads), striking volume loss shown by enlarged extra-axial space and ventriculomegaly (A, B
and C), poor gyral development with few and shallow sulci (A; long white arrows). The occipital “shelf” caused by skull collapse (C; white arrow).
Photograph source: Moore et al., 2017 (34).
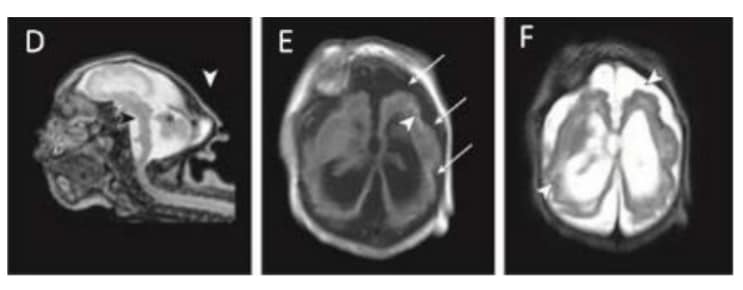
Magnetic resonance imaging (MRI) in infant with prenatal Zika exposure shows scattered punctate calcifications (E; white arrowheads), very low forehead and small cranial vault (D), striking volume loss shown by enlarged extra- axial space and ventriculomegaly (D, E and F), poor gyral development with few and shallow sulci (E; long white arrows), poor gyral development with irregular “beaded” cortex most consistent with polymicrogyria (F; white arrowheads), flattened pons and small cerebellum (D; black arrowhead and asterisk). The occipital “shelf” caused by skull collapse is seen in both infants (D; white arrowhead).
Photograph source: Moore et al., 2017 (34).
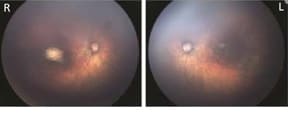
Fundus images of right and left eye: Optic nerve hypoplasia with the double-ring sign, gross pigmentary mottling, and chorioretinal scar in the macular region.
Photograph source: Moore et al., 2017 (34); Ritter et al., 2017 (35)
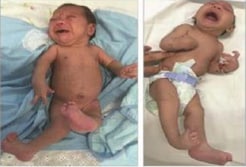
Left: Newborn infant with bilateral contractures of the hips and knees, bilateral talipes calcaneovalgus, and anterior dislocation of the knees. Right: Newborn infant with bilateral contractures of the shoulders, elbows, wrists, hips, knees, and right talipes equinovarus. Hips are bilaterally dislocated in both infants.
Photograph source: Moore et al., 2017 (34).
Diagnosis
The diagnosis of ZIKV infection is laboratory based. ZIKV RNA has been detected in blood, urine, saliva, CSF, semen and amniotic fluid, as well as tissue samples. WHO recommends testing blood and urine to diagnose ZIKV infection and, when collected for other diagnostic purposes, CSF.
Laboratory testing: Pregnant women
Women who should be tested are those living in areas of ZIKV transmission or potentially exposed to someone with ZIKV infection, and who develop rash or other symptoms consistent with possible ZIKV infection during pregnancy. In areas with co-circulation of dengue and other flaviviruses, testing for multiple arboviral infections – most notably, dengue – might also be indicated. ZIKV infection during pregnancy is confirmed by NAATs* performed on blood and/or urine collected from women generally within seven days of symptom onset. Some reports indicate that NAAT results for pregnant women might remain positive longer. Specimens with positive NAAT results should have RNA re-extracted from the same specimen and be retested by NAATs for confirmation.
* Nucleic acid amplification tests (NAATs) include, but are not limited to, reverse transcriptase polymerase chain reaction (RT-PCR), real- time RT-PCR, quantitative RT-PCR, and next-generation sequencing.
ZIKV IgM antibody testing can be performed on serum from women collected at least seven days after symptom onset. Caution should be exercised in interpretation of ZIKV IgM test results in pregnancy for two major reasons: First, currently available IgM might give false-positive results because of nonspecific reactivity or cross-reactivity among antigens found on ZIKV as well as on dengue and other flaviviruses; second, ZIKV IgM can persist up to two years; therefore, presence of ZIKV IgM might reflect infection that occurred prior to pregnancy and that does not pose a threat to the developing fetus.
Plaque reduction neutralization tests (PRNTs) are quantitative assays that measure virus-specific neutralizing antibody titres for dengue, Zika, and other flaviviruses to which the patient might have been exposed. PRNTs can resolve false-positive IgM antibody results caused by nonspecific reactivity and, in certain cases, can help identify the infecting virus. In primary flavivirus infections, a neutralizing antibody titre that is at least fourfold higher than titres against other flaviviruses to which the person might have been exposed usually determines the specific infecting flavivirus. However, PRNT testing has been available in limited settings to date.
The majority of ZIKV infections are asymptomatic. Asymptomatic ZIKV infections during pregnancy can result in CZS and other adverse pregnancy outcomes. Testing asymptomatic pregnant women by NAATs is recommended for outbreak settings as the interpretation of serologic results can be challenging due to the increased risk of false-positive test results. There are no perfect tests for ZIKV. NAATs have a narrow window of identification of ZIKV RNA (< 7 days in blood and 14 days in urine), and currently available IgM assays have the limitations described above. Proper confirmation of positive test results in asymptomatic pregnant women is of particular importance. The WHO case definition for ZIKV infection is presented in Table 5.2.
Table 5.2. WHO case definition for ZIKV infection for pregnant women and general population
| Suspected case | A person presenting with rash and/or fever and at least one of the following signs or symptoms:
|
| Probable case | A suspected case with presence of IgM antibody against ZIKVa and an epidemiological linkb. |
| Confirmed case | A person with laboratory confirmation of recent ZIKV infection:
|
a No evidence of other flavivirus infection.
b Sexual or bloodborne contact with a confirmed case or a history of residing in or travelling to an area with local transmission of ZIKV within two weeks prior to onset of symptoms.
Laboratory testing: Women and infants after delivery
Suspected cases of CZS based on prenatal and postnatal detection of possible Zika-related birth defects should be investigated for evidence of ZIKV infection, including laboratory testing and clinical examination of liveborn infants, stillbirths and fetal deaths, although Zika-related birth defects are difficult to prenatally detect in early fetal losses. ZIKV RNA has been detected for extended periods of time in affected infants. Specimens collected from mothers and liveborn infants should be tested for ZIKV RNA (blood, urine) and IgM (serum). Tissue recovered from fetal deaths and stillbirths might also be tested for ZIKV RNA. Cord blood should not be used because positive results might reflect contamination with maternal blood and are not indicative of congenital infection. Because the possibility of the newborn having had a previous flavivirus infection is low, and IgM does not typically cross the placental barrier, detection of IgM antibodies against ZIKV in neonatal serum constitutes an important finding indicative of intrauterine infection. Molecular analysis (NAATs) and the detection of anti- ZIKV IgM antibodies by enzyme-linked immunosorbent assay (ELISA) can also be performed in a CSF sample obtained by medical indication for the diagnosis of the congenital syndrome, but is not exclusively used for diagnosing ZIKV infection. Positive test results indicate a confirmed or probable case of CZS; however, the sensitivity and specificity of infant testing has not been determined and negative test results do not definitively rule out CZS. Other factors to take note of include:
- NAATs should be performed on blood and/or urine collected from patients within seven days of symptom onset.
- IgM antibody testing should be performed on blood from patients at least seven days after symptom onset.
- If available, NAAT or IgM antibody testing can be performed on CSF.
- Whenever possible, paired serum specimens should be collected at least two to three weeks apart, ideally with the first serum specimen collected during the first five days of illness.
- Patients should be tested for infection with ZIKV in addition to dengue and other circulating flaviviruses if applicable, and chikungunya virus either sequentially or in parallel if specifically suspected based on symptoms or epidemiology.
Radiology
Assessment for brain anomalies due to congenital ZIKV infection should be performed for all infants with known or suspected exposure. Initial investigation using head ultrasound can reveal decreased brain parenchyma associated with loss of cortical tissue, midline defects such as agenesis of the corpus callosum, increased fluid collection in the form of hydrocephalus ex vacuo and, less reliably, cortical malformations such as pachygyria and peripherally located calcifications between the cortical and subcortical layers. With microcephaly and a small anterior fontanelle, views of the periphery as well as the posterior fossa might be limited. Demonstration of calcifications is optimally obtained by CT scan and characterization of cortical malformations by MRI.
Prenatal imaging by ultrasound or MRI can detect Zika-related brain malformations; however, the sensitivity and specificity of prenatal ultrasound is not known. Microcephaly might not be detected prenatally until late-second or early-third trimesters. Prenatally diagnosed abnormalities should be confirmed by CT scan or MRI after birth. Case definitions for ZIKV infection are presented in Table 5.3.
Table 5.3. Case definition for ZIKV infection
| Suspected case of congenital syndrome associated with asymptomatic maternal ZIKV infection |
|
| Probable case of congenital syndrome associated with symptomatic maternal ZIKV infection |
|
| Confirmed case of congenital syndrome associated with ZIKV infection |
|
a Excludes early brain malformations such as NTDs and holoprosencephaly.
b See main clinical manifestations in the infant (p. 162).
Fig. 5.6. Testing in suspected cases of ZIKV infection
Molecular testing using a multiplex NAAT in suspected cases of ZIKV infection in areas with circulation of other arboviruses
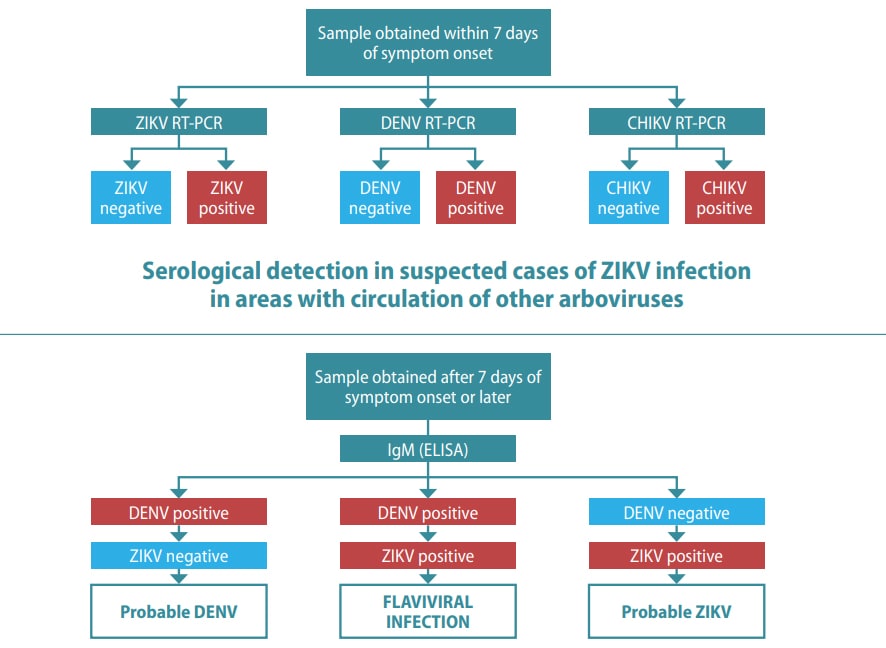
CHIKV: chikungunya virus; DENV: dengue virus; ELISA: enzyme-linked immunosorbent assay; IgM: immunoglobulin M; neg: negative; pos: positive; RT-PCR: reverse transcriptase polymerase chain reaction; ZIKV: Zika virus
Relevant ICD-10 codes
P35.8 Other congenital viral diseases
Q02 Microcephaly
Checklist
- Examine the neonate for:
- Severe microcephaly with collapsed skull and redundant scalp.
- Congenital contractures of major joints (arthrogryposis).
- Hypertonia and spasticity.
- Macular scarring and focal pigmentary retinal mottling (paediatrician or ophthalmologist).
- Additional clinical examinations:
- Radiographs: Thin cerebral cortices with subcortical calcifications.
- Hearing assessment: Screening by auditory brain stem response methodology with diagnostic testing to verify hearing loss.
- Obtain maternal medical health and pregnancy history to ascertain ZIKV infection.
- Collect maternal and neonatal samples for diagnostic testing.
- Determine whether the case is suspected, probable or confirmed.
- Obtain photographs of the malformations observed.
- Record diagnostic information and report.