September 2018 LTCF Newsletter
September 2018 Newsletter – Print version [PDF – 3 MB]
Recap: 2018 NHSN LTCF Component Training
The 2018 NHSN Long-term Care Facility Component Annual Training kicked off on a high note!
Dr. Daniel Pollock, MD, NHSN Surveillance Branch Chief, welcomed participants by describing the Centers for Disease Control and Prevention’s (CDC) history in surveillance and how the National Healthcare Safety Network (NHSN) was established. Dr. Jeneita Bell, MD, NHSN Long-term Care Lead, followed with a presentation on the national perspective of infection surveillance in LTC. During the two-and-half day session, other CDC staff shared information regarding infection prevention, reporting, and data analysis. In addition, two external speakers shared their experience with NHSN and quality improvement implementation. By the end of the training, over 1,000 in-person and virtual participants were educated on infection prevention and the LTCF Component.
We are thankful to The National Association of Directors of Nursing Administration in Long-term Care (NADONA) for their generous donation and provision of a sweet and much appreciated surprise for the nearly 100 onsite participants.
Again, thank you for making our 2018 NHSN Long-term Care Facility Component Annual Training a success! We recognize that your participation and commitment to improve resident health outcomes are critical to advancing our mission.
We value your continued support!
– The LTC Team
Now Available! 2018 NHSN LTCF Training Presentations
The 2018 NHSN Long-term Care Facility Component Annual Training videos and slide decks are now available on the NHSN website!
Recorded presentations include 2018 NHSN updates, epidemiology and infection surveillance in long-term care, and more. Recorded sessions covering validation of NHSN data and antibiotic stewardship surveillance practices are also available.
All videos and slide PDFs are located on the NHSN training page.
Continuing Education (CE) is available for this activity. Information on how to obtain Continuing Education for NHSN Training Events, can be found here.
Director’s Recognition Award: Training on Antibiotic Stewardship Team

After CDC’s Division of Healthcare Quality Promotion (DHQP) understood the need for training on appropriate antibiotic use, the DHQP Antibiotic Stewardship Team produced a comprehensive program based on the latest science. CDC Training on Antibiotic Stewardship, an interactive online course that offers participants up to eight hours of free continuing education, serves as a resource for a wide array of stakeholders and helps learners deliver effective and consistent messages to patients about antibiotic use and antibiotic resistance. Within a matter of weeks, 4,500 people have registered to take the course. The Antibiotic Stewardship Team was recognized for this accomplishment (left). Register to take the course today!
Prevention Process Measures Module
Unclean hands of healthcare workers and environmental contamination play a significant role in the spread of communicable diseases among residents in Long-Term Care facilities (LTCF). What is your facility doing to prevent the spread of communicable diseases? How do you measure the effectiveness of your prevention strategies?
The CMS – Medicare and Medicaid; Reform of Requirements for Long-Term Care Facilities (Final Rule 483.80) require that LTCFs establish a system of surveillance designed to identify possible communicable diseases or infections. NHSN’s LTCF Component offers facilities several surveillance options. One of these options is the Prevention Process Measures Module. This surveillance module provides facilities with a method to document and analyze adherence to prevention practices, including hand hygiene after contact with a resident or surfaces in immediate vicinity of the resident; and gown and glove use in the context of transmission based precautions. By documenting prevention process measures, facilities are able to identify potential sources of infection transmission, opportunities for improvement, and measure the progress of prevention efforts at a facility level.
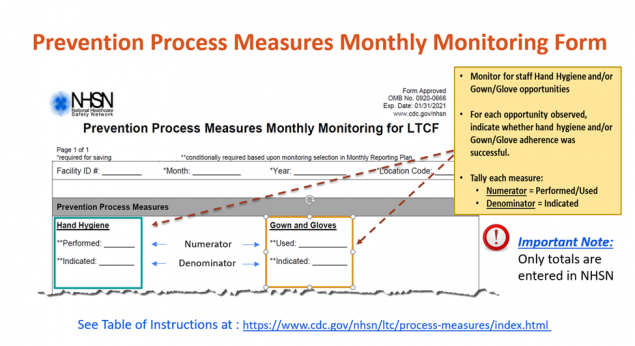
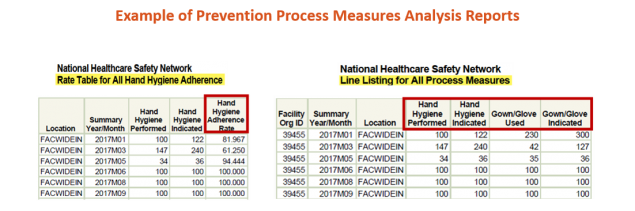
Please contact us at nhsn@cdc.gov and include “LTCF – Prevention Process Measures” in the subject line, if you have additional questions or need assistance with enrolling or adding this module to your Monthly Reporting Plan. We are happy to assist!
LTCF Data Validation Guidance
The 2018 NHSN LTCF Data Validation Guidance and Toolkit for C. difficile infection (CDI) is now posted to the NHSN website. This guidance provides methods for validating CDI LabID Events in Long-term Care Facilities (LTCF). Performing internal or external validation activities will assure high-quality surveillance data through accountability and by identifying, understanding, and correcting reporting problems. The intended audience for this external guidance is state health departments and other oversight agencies seeking to validate performance and adherence to the 2018 LTCF CDI LabID Event protocol.
Test Your Knowledge
Question 1: I am trying to report a CA-SUTI to NHSN, but the Specific Event box is grayed out. What am I doing wrong?
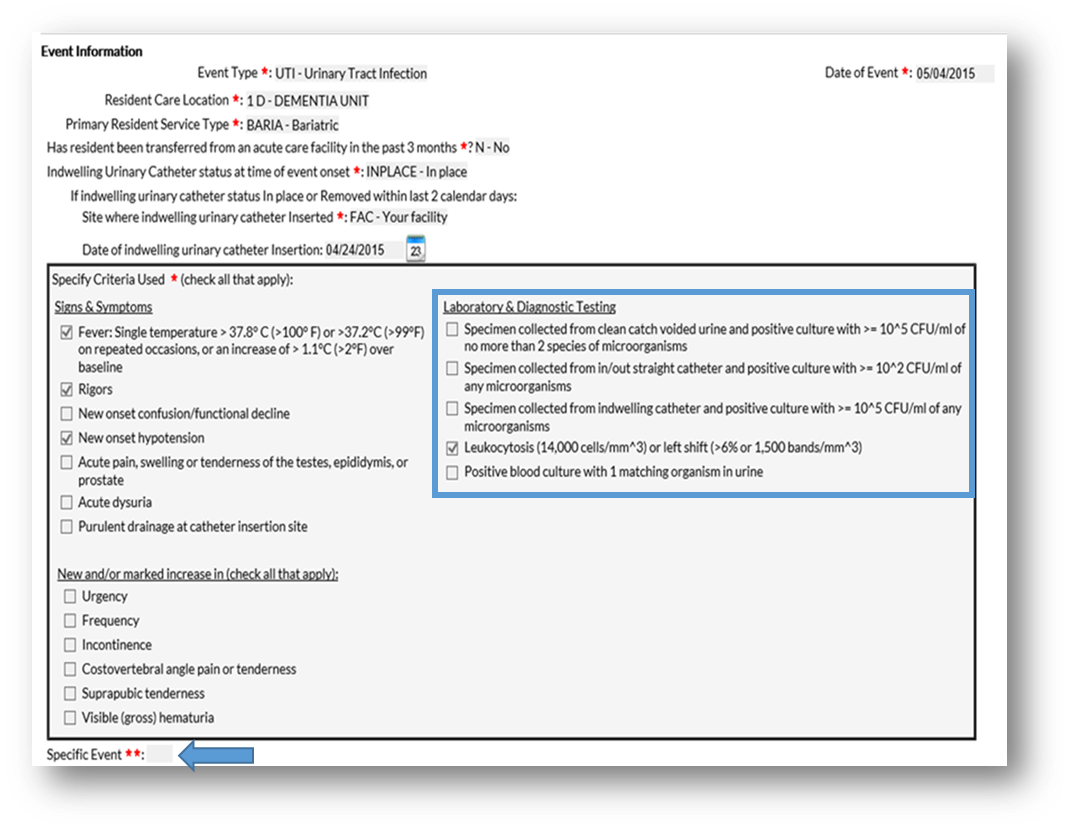
- You did not select the correct signs and symptoms to meet the NHSN CA-SUTI criteria
- You did not select the required urine culture result to meet the NHSN CA-SUTI criteria
- You must manually type in the Specific Event
Answer: The correct answer is B. You did not select the required urine culture results to meet the NHSN CA-SUTI criteria.
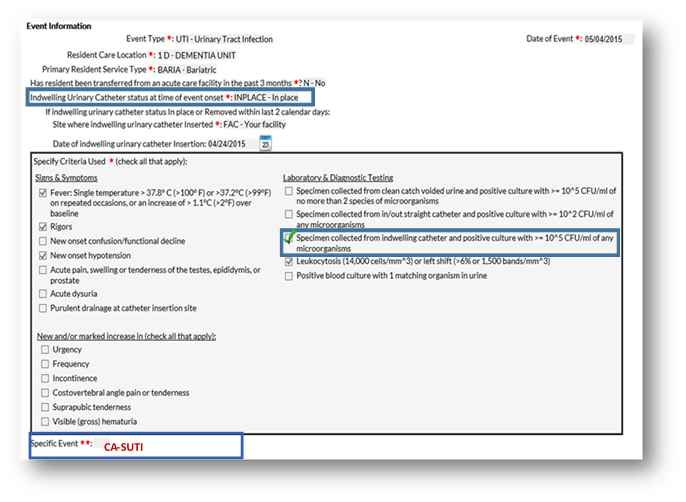
Rationale:
- A qualifying positive urine culture is required to meet NHSN UTI criteria
- The correct NHSN UTI criteria must be selected before the NHSN application will auto-populate the SPECIFIC EVENT TYPE
- If the resident does not meet the NHSN UTI criteria, then a UTI event should not be submitted to NHSN
Question 2: Should you report the specimen collected in the Emergency Department (ED) as a CDI LabID Event for the LTCF?
Case: Mr. A is a resident in your LTCF. He does not have a history of C. difficile. On March 1, he was transferred to the local emergency department (ED) for evaluation of diarrhea and fever. While in the ED, a loose stool specimen tested positive for C. difficile. He received IV fluids and was transferred back to the LTCF in a contact isolation room on March 2. Should the LTCF report a CDI LabID Event from Mr. A?
- YES
- NO
Answer: A
Rationale: Since the specimen was collected in an outpatient location (ED) and Mr. A returned back to the LTCF within 2 calendar days of leaving, the positive C. difficile specimen was entered into NHSN as a CDI LabID Event for the LTCF. When entering the event, the location of the resident should represent the location of the resident prior to transfer to the OP facility.