FAQs: Multidrug-Resistant Organism & Clostridioides difficile Infection (MDRO & CDI)
On This Page
- Numerator Reporting for LabID Events: Definition of CDI Assay
- Numerator Reporting for LabID Events: Testing for CDI
- Numerator Reporting for LabID Events: Acceptable specimens for CDI reporting
- Denominator Reporting for LabID Events: Standard Testing Method for CDI
- Numerator Reporting for LabID Event: Transfer rule
- Numerator Reporting for LabID Event: Discharged in past 4 weeks
- Numerator Reporting for LabID Event: Prior evidence of infection
- Numerator Reporting for LabID Event: Admission date for inpatient rehabilitation facilities (IRF)
- Numerator Reporting for LabID Event: MRSA bacteremia, all specimen source
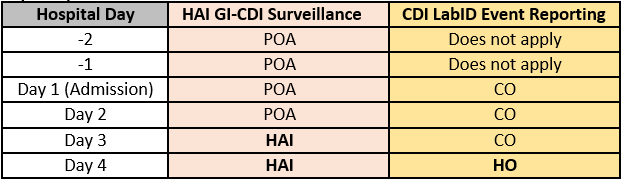
- Distinguishing healthcare-associated infection (HAI) and LabID events
- Denominator Reporting for LabID Events: Outpatient encounter
- Denominator Reporting for LabID Event: Facility count
- Denominator Reporting for LabID Event: Report No Events
- Categorizations: History of CDI
- Categorizations: Categories of the cdiAssay variable (Recurrent, incident, and blank)
- Locations: Swing beds & observation patients
- Analysis: SIR
- Analysis: Line listing, indicator variable
- Analysis: Line listing, categorizations of MRSA bacteremia LabID Events
- CMS Inpatient Quality Reporting (IQR) Program for Acute Care Hospitals (ACH): CMS reporting requirements & data submitted