Using the SAAR to Track Impact of Multiple Concurrent Antimicrobial Stewardship Interventions
Key Take Away Points
- An academic medical center used changes in its NHSN Antimicrobial Use (AU) Option Standardized Antimicrobial Administration Ratio (SAAR) to evaluate the impact of multiple antimicrobial stewardship interventions.
- Prospective audit and feedback data suggested overuse of levofloxacin for oral step-down therapy in community-acquired pneumonia.
- Review of source-specific antibiogram data indicated that a change was needed in the local recommendations for empiric therapy of urinary tract infections from ceftriaxone to cefazolin.
- Tracking relevant SAAR categories allowed the Antimicrobial Stewardship program to monitor the impact of these two interventions.
Northwestern Memorial Hospital (NMH), located in downtown Chicago, is an 894 bed academic medical center and the primary teaching affiliate for Northwestern University Feinberg School of Medicine. It is the flagship hospital of the Northwestern Medicine system. The Antimicrobial Stewardship program consists of a team of Infectious Diseases (ID)-trained pharmacists and ID physicians.
The Need
During daily prospective audit and feedback, antimicrobial stewardship team members identified that community-acquired pneumonia patients, despite receiving intravenous beta-lactam/macrolide therapy initially, were routinely switched to levofloxacin for step-down to oral therapy.
Additionally, previous NMH urinary tract infection treatment guidelines suggested that for patients who required intravenous therapy, ceftriaxone was the antibiotic of choice. Review of the most recent antibiogram data suggested that cefazolin provided near-equivalent coverage of the most common urinary pathogens.
The Interventions
Community-acquired pneumonia step-down to oral therapy guidelines were developed to emphasize the guideline recommendations to step-down to the same class of antibiotics that the patient received intravenously. The guidelines were then disseminated in table form to prescribers and clinical pharmacists. Recommendations were reinforced by daily audit and feedback review as well as interventions by unit-based clinical pharmacists.
Urinary tract infection guidelines were revised to recommend cefazolin as the first option to treat hospitalized patients without a history of multi-drug resistant organisms. This was reinforced by both daily audit and feedback review as well as interventions by unit-based clinical pharmacists.
Because both interventions, implemented in January of 2018, were designed to reduce prescribing of antibiotics in the broad spectrum antibacterial agents used predominately for community-acquired infections (BSCA) SAAR group and move prescribers to choose agents in the narrow spectrum beta-lactam (NSBL) SAAR group, we tracked the impact on these two metrics.
The Results
After implementation of the interventions on internal medicine units, a substantial rise in the NSBL SAAR was realized with a corresponding decrease in the BSCA SAAR (Figure).
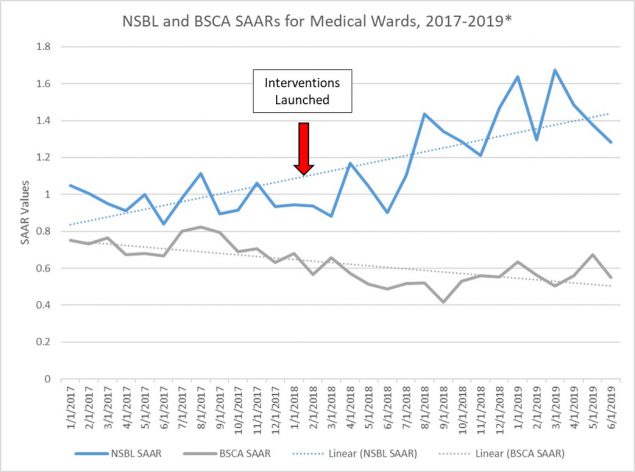
*Trend lines shown here are for visualization purposes only and do not provide insight into whether observed changes in SAAR values over time are statistically significant.
These data have been reported to the Department of Medicine Quality Committee and are presented quarterly to the Antimicrobial Subcommittee of the Pharmacy and Therapeutics Committee.
For more information contact: Michael Postelnick, RPh BCPS AQ ID
Antimicrobial Stewardship Coordinator
Northwestern Memorial Hospital
Note: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.