December 2018 LTCF Newsletter
- Save the Date: 2019 NHSN LTCF Component Training
- Long-Term Care Facility Component Annual Facility Survey
- Upcoming Webinars
- NHSN LTCF Reporting & Surveillance Home Page
- 2019 NHSN LTCF Component Updates
- What to do if you missed the Re-Consent Deadline
- Common Enrollment Errors
- AMDA’s 2019 Annual Conference
- Share Your Stories
- We’d Love to Hear From You...
December 2018 Newsletter – Print version [PDF – 2 MB]
Save the Date: 2019 NHSN LTCF Component Training
Plan to join us July 9-11, 2019 for the 2019 National Healthcare Safety Network’s (NHSN) Annual Training for Long-term Care Facilities. The training will be held on the CDC campus in Atlanta, Georgia and will cover various topics such as antibiotic stewardship, data analysis, and surveillance for urinary tract infections, C. difficile, and multi-drug resistant organisms. Please email NHSNtrain@cdc.gov with training-related questions.
Long-Term Care Facility Component Annual Facility Survey
Be on the lookout for communications about the release of the 2018 LTCF Component Annual Facility Survey. The survey will open in January and must be completed prior to March 1, 2019 to avoid losing the ability to submit event data to NHSN. Unless otherwise specified in the survey, the data reported will include facility data and practices during January 1, 2018 through December 31, 2018. If your facility completed a 2017 survey, it may be helpful to print a copy of the completed 2017 annual facility survey for reference while completing the 2018 survey.
Additionally, users are encouraged to complete the paper version of the 2018 survey form [PDF – 100 KB] prior to entering the information into the web application because incomplete surveys cannot be saved. Instructions for completing the survey are located in the Table of Instructions [PDF – 500 KB] document under the Data Collection Forms tab on the LTCF website. For questions, please e-mail the NHSN helpdesk at nhsn@cdc.gov with “LTCF Annual Survey “in the subject line.
Upcoming Webinars
Join us online for an educational webinar in which we will review important tips for completing the NHSN Annual Facility Survey for Long-term Care Facilities, as well as discuss updates for the 2019 calendar year. Participants will have time to ask questions. To accommodate diverse schedules, the webinar will be offered twice — Wednesday, January 9 – 1:00-2:00 pm EST and Wednesday, February 6 – 1:30-2:30 pm EST. Watch your e-mail for an invitation to register.
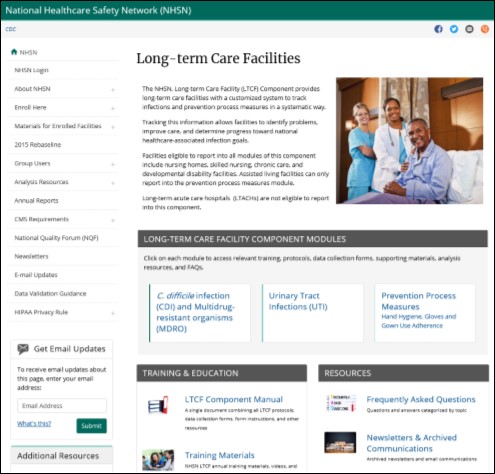
NHSN LTCF Reporting & Surveillance Home Page
We‘ve listened to your feedback and have designed a new long-term care facility webpage to enhance the usability, design and accessibility of the NHSN website. Some of the enhancements we’ve made include: renaming and highlighting the modules that long-term care facilities are eligible to report into; easy access to the LTCF Component Manual and Frequently Asked Questions; and more. We anticipate the updated website to be released in late December or early January.
2019 NHSN LTCF Component Updates
It’s that time of the year again! Below you will find a summary of the NHSN LTCF Component updates coming in January 2019. Keep in mind, any changes to NHSN surveillance criteria or denominator data collection requirements will go into effect beginning on January 1, 2019.
- New required variable called “CDI Treatment Starts” added to the Monthly Summary Data /Denominators for LTCF forms to enable an estimate of CDI burden in a facility when empiric treatment for CDI occurs in the absence of confirmatory testing.
Note: Facilities will be required to report this additional variable beginning with January 2019 summary data/denominator submission. - To improve data quality on the LTCF Annual Facility Survey, a pop-up message will appear as a reminder to verify the primary testing method for C. difficile when:
- An uncommon C. difficile testing method is selected (specifically, culture or cell cytotoxicity neutralization assay) OR
- “Other” is selected and the testing method that is manually typed in the space is equivalent to one of the provided testing methods.
- To improve data quality on Event reporting, a pop-up message will appear on the Event Page if the selected Resident Type (Short Stay [SS] or Long Stay [LS]) does not meet the NHSN definition based on the date of first admission and the event date.
- The following changes were made to the urine culture requirements for UTI Event type:
- Specimen collected from in/out straight catheter and a positive culture with no more than 2 species of microorganisms, at least one of which is a bacterium of ≥ 105 CFU/ml
- Specimen collected from indwelling catheter and a positive culture with no more than 2 species of microorganisms, at least one of which is a bacterium of ≥ 105 CFU/ml
Note: Facilities will be required to use the updated urine culture requirements to meet NHSN UTI criteria beginning with UTIs identified on January 1, 2019 and forward.
- In the NHSN line listing and rate tables, the column titles were updated to reflect the descriptive variable names as the default instead of the variable names.
- The following additional variables added as columns to the default Line Listing – All CDI LabID Events: (1) CDI Assay; (2) Onset; (3) Onset Description; and (4) Days: Admit to Event. Definitions for incident and recurrent CDI added as footnotes to Line Listing – All CDI LabID Events.
- Clostridium difficile infection (CDI), also known as C. difficile infection, has been reclassified as Clostridioides difficile (CDI), also known as C. difficile infection. While this update has been made in the NHSN protocols, forms, and table of instructions, the update will not be recognized in the NHSN interface until a later date.
What to do if you missed the Re-Consent Deadline
If your facility has missed the July 2018 deadline to re-consent, your NHSN account is temporarily suspended. Please DO NOT attempt to re-enroll to NHSN or re-register with SAMS. Instead, to re-gain access to NHSN, complete the following steps:
- The NHSN facility administrator or NHSN component Primary Contact User must log in and accept the re-consent forms.
- If you do not know the facility primary contact/ administrator, email the user support team at NHSN@cdc.gov. Please add “LTC re-consent form” in the subject line so that your message reaches the appropriate person.
Common Enrollment Errors
We have notice that some facilities have enrolled more than once in NHSN. As a reminder, you DO NOT need to re-enroll under any circumstance. If you notice that your facility has more than one OrgID, please contact user support at NHSN@CDC.gov and include “LTC duplicate facility” in the subject line. You may also contact user support if you need to add a new user, change your facility name, or change the facility CCN.
AMDA’s 2019 Annual Conference
Registration is underway for the 2019 AMDA— The Society for Post Acute and Long-Term Care Medicine Annual Conference. It will be taking place March 7-10, 2019 in Atlanta Georgia. Attendees are exposed to the latest clinical developments in geriatric medicine, best practices in medical direction, policy/regulatory updates, as well as practical tools to help implement new ideas in your practice setting.
During the conference, a few CDC staff will be presenting the AMDA/CDC/SHEA Infection Prevention in PALTC Certificate Course, Thursday, March 7, 2019.
Presentations during this time period:
- Transmission of Infectious Agents in Healthcare Settings – Robin Jump, MD, PhD
- Managing Outbreaks – Theresa Rowe, DO, MS
- Strengthening Infection Prevention and Antibiotic Stewardship at Care Transitions – Ghinwa Dumyati, MD
- Panel Discussion: Challenging Situations – Nimalie Stone, MD, MS (Moderator); Laurie Archbald-Pannone, MD, MPH; Ghinwa Dumyati, MD; Robin Jump, MD, PhD; Sarah Kabbani, MD, MSc ; David Nace, MD, MPH, CMD; Theresa Rowe, DO, MS
Summary: Developed in partnership with the Society for Healthcare Epidemiology of America (SHEA) and the Centers for Disease Control and Prevention (CDC), the course will address aspects of infection control and prevention that are unique to skilled nursing facilities, which must balance a home-like environment while minimizing the risk of infections. The course will review occupational health concerns in the PALTC setting, including outbreaks that affect both staff and residents, opportunities to improve antimicrobial use, and strategies to reduce the transmission of infections such as multi-drug resistant organisms and Clostridium difficile.
Share Your Stories
We would love for you to share your stories about what’s happening in the field of healthcare and long-term care resident safety. Do you have any success stories relating to science or research in the works? If you’ve been a part of a successful program or initiative that has helped nursing homes improve infection prevention, healthcare quality or surveillance, then we want to hear from you! For more information or questions, please email nhsn@cdc.gov and include “LTCF stories” in the subject line.
We’d Love to Hear From You…
- Is your organization struggling with promoting the benefits of the National Healthcare Safety Network (NHSN) and surveillance reporting?
- Does your infection prevention staff need training and technical assistance on the NHSN Long-term Care Facility Protocols?
- Are there any topics you would like for us to host a webinar on?
- Do you know how to view, print, interpret, and discuss your NHSN data reports?
- Do you have ideas for data reports you’d like to see available in NHSN?
- Has your LTC facility been successful in reporting data to NHSN?
- How have you used NHSN in your prevention efforts?
- How can we help you to put your data into action?
We can do more together! Help us create opportunities for learning and exchanging Best Practices. Submit your requests and comments to nhsn@cdc.gov and include “LTCF Technical assistance” in the subject line of the email.