December 2019 LTCF Newsletter
December 2019 Newsletter – Print version [PDF – 1 MB]
LTCF Component 2019 Annual Facility Survey
Be on the lookout for communications about the release of the 2019 LTCF Component Annual Facility Survey. The survey will open in January and must be completed prior to March 1, 2020. After March 1st, facilities will be unable to enter new monthly reporting plans until required Annual Facility Surveys are completed. Unless otherwise specified, items in the survey pertain to facility characteristic and practices during January 1, 2019 through December 31, 2019.
If your facility completed a 2018 survey, it may be helpful to print a copy of the completed 2018 annual facility survey for reference while completing the 2019 survey. No changes were made to the 2019 LTCF annual survey. Additionally, users are encouraged to complete the paper version of the 2019 survey form prior to entering the information into the web application because incomplete surveys cannot be saved. Instructions for completing the survey are located in the Table of Instructions document under the Data Collection Forms tab on the LTCF website.
For questions, please e-mail the NHSN helpdesk at nhsn@cdc.gov with “LTCF Annual Survey “in the subject line.
Upcoming Webinars
Join us online for an educational webinar in which we will review important tips for completing the NHSN Annual Facility Survey for Long-term Care Facilities, as well as discuss updates for the 2020 calendar year. Participants will have time to ask questions. To accommodate diverse schedules, the webinar will be offered twice —Wednesday, January 8 – 1:30-2:30 pm EST and Wednesday, February 5 – 1:30-2:30 pm EST. Watch your e-mail for an invitation to register.
2020 NHSN LTCF Component Updates
It’s that time of the year again! Below you will find a summary of the NHSN LTCF Component updates coming in January 2020. Keep in mind, any changes to NHSN surveillance criteria or data collection requirements will go into effect beginning on January 1, 2020.
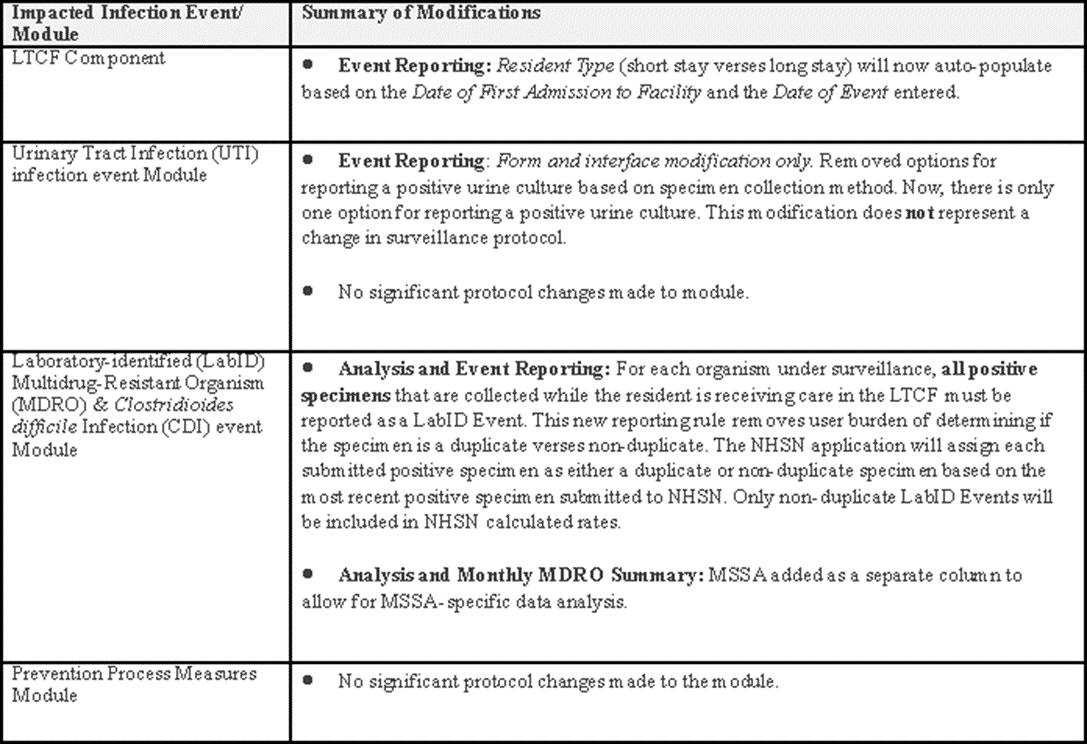
The most significant change will be in the LabID Event Module. All positive results from specimens collected while the resident was receiving care within the LTCF must be reported for the participating events (C. difficile and individual MDROs). The purpose of this change is to reduce the burden on users for determining which events are considered as a reportable non-duplicate LabID event. NHSN will remove the duplicate specimens from calculated rates. Please join one of our upcoming webinars and see the updated LabID Event protocol for additional information and case scenarios.
How to Report Healthcare Personnel Influenza Vaccination Summary Data
Long-term care facilities are encouraged to use the following steps to voluntarily report healthcare personnel (HCP) influenza vaccination summary data through NHSN for the 2019-2020 influenza season.
Activate the Healthcare Personnel Safety (HPS) Component in NHSN
1.) NHSN Facility Administrator logs into SAMS:
- Click ‘NHSN Reporting’.
2.) From the Home Page, click ‘Facility’ then ‘Add/Edit Component’.
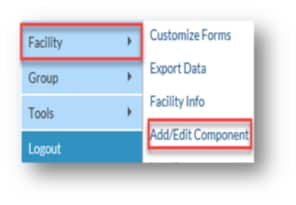
- Check box next to Healthcare Personnel Safety.
- Click ‘Save,’ if applicable.
3.) Facility Administrator adds HPS Component Primary Contact.
- Enter name, phone, e-mail, and address for person to be contacted if CDC/NHSN has updates or questions about the HPS Component.
- Click ‘Save’.
4.) NHSN Facility Administrator adds HPS Component Primary Contact as a user
(if not already a facility user) within the NHSN facility.
- Click ‘Users’ on the navigation bar, then click ‘Add’.
- Complete ‘Add User’ screen mandatory fields.
- Click ‘Save,’ if applicable.
Create Monthly Reporting Plan
1.) Login to the HPS Component of NHSN.
2.) Click on ‘Reporting Plan’ and then ‘Add’ on the left-hand navigation bar.
3.) Select ‘March 2020’ for your plan, if reporting for the 2019-2020 influenza season. (Note: If you see a pop-up box stating: ‘No data found for March 2020,’ you can simply click ‘OK’ and proceed to the next step).
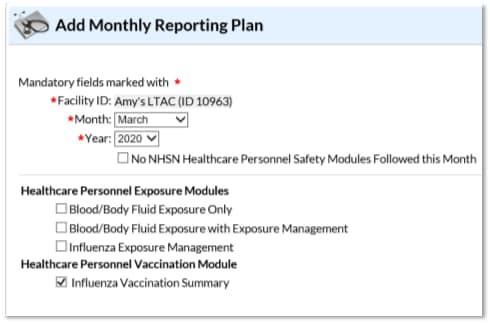
4.) Check the box next to ‘Influenza Vaccination Summary’ under the ‘Healthcare Personnel Vaccination Module’
5.) Click ‘Save’
Enter Data
1.) Go to ‘Flu Summary’ and then ‘Add’ on the left-hand navigation bar.
2.) Click ‘Continue’ to proceed as ‘Influenza Vaccination Summary’ appears as the default option on the drop-down menu.
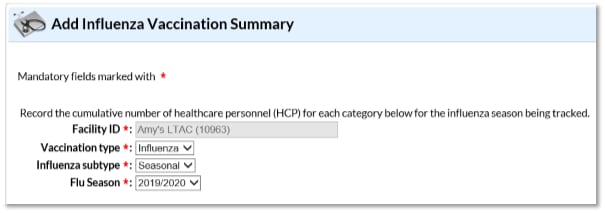
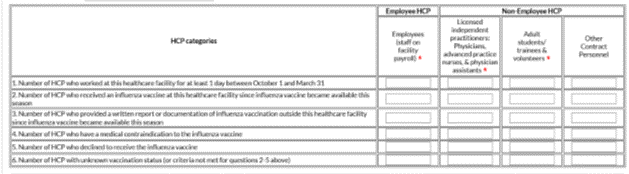
3.) You will now see the data entry screen for the HCP influenza vaccination data summary. Select the ‘2019-2020’ influenza season for the ‘Flu Season’ drop-down box.
4.) After you have finished entering your data, save the data by clicking the ‘Save’ button at the bottom of the screen. You should then see a message at the top of your screen confirming that your data have been saved.
Resources:
- NHSN Help Desk: nhsn@cdc.gov (Please include ‘HPS Flu Summary’ and ‘Long-term care facility’ in the e-mail subject line).
- NHSN Training Slides [PDF – 2 MB].
Enhanced Barrier Precautions: New Guidance for Nursing Homes
With the need for an effective response to the detection of serious antibiotic resistance threats in nursing homes, CDC recently released new guidance on the Implementation of Personal Protective Equipment (PPE) in nursing homes to prevent the spread of novel or targeted multidrug-resistant organisms (MDROs). A new approach to gown and glove use during resident care, called Enhanced Barrier Precautions (EBP) is introduced in this guidance. Enhanced Barrier Precautions expand the use of PPE beyond situations in which exposure to blood and body fluid is anticipated and refer to the use of gown and gloves during high-contact resident care activities, such as assisting with bathing or dressing, that provide opportunities for transfer of MDROs to staff hands and clothing.
EBP is indicated for residents with novel or targeted MDROs including pan-resistant organisms, Carbapenemase-producing organisms, and Candida auris. In addition, EBP is indicated for residents with indwelling medical devices and wounds, who are at high risk for acquiring and being colonized with MDROs, when they reside on the same unit as a resident colonized or infected with a novel or targeted MDRO.
Resources:
- Check out the Enhanced Barrier Precautions guidance including a description of high-contact resident-care activities and recommendations for implementation of EBP.
- A recent webinar given on Enhanced Barrier Precautions.
- Example signage for Enhanced Barrier Precautions [PDF – 1 MB] and Transmission-Based Precautions.
Did You Know?
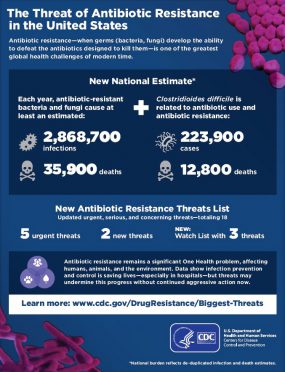
Antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and 35,000 deaths in the United States each year—that’s at least one infection every 11 seconds and one death from antibiotic resistance every 15 minutes.
In CDC’s recent Antibiotic Resistance Threats in the United States, 2019 report, Carbapenem-resistant Acinetobacter (ACINE), Carbapenem-resistant Enterobacteriaceae (CRE), and Clostridioides difficile (C. difficile) are classified as urgent antibiotic resistance threats. These are also reportable events in the NHSN LabID (CDI/MDRO reporting) reporting module for LTCFs.
Enroll in the LabID module to monitor these urgent antibiotic resistance threats in your facility. Also enroll in the Prevention Process Measures to see how well your staff implement strategies to prevent these deadly bacteria from spreading in your facility.
Infographic:
The Threat of Antibiotic Resistance in the United States [PDF – 1 MB]
We’d Love To Hear From You…
- Is your organization struggling with promoting the benefits of the National Healthcare Safety Network (NHSN) and surveillance reporting?
- Does your infection prevention staff need training and technical assistance on the NHSN Long-term Care Facility Protocols?
- Are there any topics you would like for us to host a webinar on?
- Do you know how to view, print, interpret, and discuss your NHSN data reports?
- Do you have ideas for data reports you’d like to see available in NHSN?
- Has your LTC facility been successful in reporting data to NHSN?
- How have you used NHSN in your prevention efforts?
- How can we help you to put your data into action?
We can do more together! Help us create opportunities for learning and exchanging Best Practices. Submit your requests and comments to nhsn@cdc.gov and include “LTCF Technical assistance” in the subject line of the email.