FAQs: Bloodstream Infection (BSI) Events
- Secondary BSI
- Site-specific criteria and secondary BSI
- Secondary BSI to lower respiratory events in locations performing VAE surveillance
- Secondary BSI assignment to a PNEU event
- Secondary BSI Assignment to a GIT 1b or IAB 2b when organisms are identified on histopathologic exam.
- Capturing non-matching organisms in a positive blood specimen
- MBI-LCBI vs. Secondary BSI
- MBI-LCBI Reporting
- Distinguishing serial reportable infections from single, unresolved infection
- Device association during the BSI repeat infection timeframe (RIT)
- Blood culture collection methods
- Defining "separate occasions"
- Common commensals are a single element
- Matching Common Commensals
- Central line counts for device attribution and denominator data
- Removal and reinsertion of a central line
- Midline catheter
- Catheter tips
- Multiple central lines
- Pus or purulence at the vascular access site
- Intra-aortic balloon pumps (IABP)
- Femoral arterial lines
- Dialysis patients
- Contracted staff
- Patient suspected of injecting into vascular catheter
- Vital Signs (hypotension, apnea, bradycardia)
- Reporting Locations
- Submitting a BSI case review to NHSN
- Central Line Associated Bloodstream Infection (CLABSI) Exclusions
- MBI RIT Exception
- Non-culture Based Testing Method (NCT)
Secondary BSI
If you believe an LCBI is secondary to a non-blood source of infection, you must first fully meet one of the NHSN site-specific infection definitions as defined in Chapter 17 (CDC/NHSN Surveillance Definitions for Specific Types of Infections), or the PNEU, UTI, or SSI protocols. Once you have done this, apply the Appendix B guidelines (Secondary BSI Guide) located in Chapter 4 [PDF – 900 KB] of the NHSN Patient Safety Component Manual.
There are only 2 scenarios in which a BSI can be deemed secondary to another site- specific infection for NHSN reporting purposes:
- The blood specimen and primary site-specific specimen (used to meet the primary infection criteria) must have at least one matching organism, AND the collection date of the blood specimen is within the primary site-specific infections secondary BSI attribution period (Scenario #1).
OR
- The blood specimen must be an element used to meet the site-specific infection criterion and be collected in the site-specific infection window period (Scenario #2).
The rules for the infection window period and secondary BSI attribution period always apply. More guidance about these rules can be found in Chapter 2 (Identifying HAIs in NHSN) [PDF – 1 MB].
Additionally, a BSI may also be secondary to VAE following the guidance outlined in the VAE protocol [PDF – 2 MB].
NOTE: If the patient does not meet any of the site-specific infection criteria from PNEU, UTI, SSI, VAE, or any of the definitions in Chapter 17 and does not meet the requirements for secondary BSI, then the LCBI must be reported as a primary LCBI and as a CLABSI if an eligible central line is present on the BSI date of event or the day before.
Site-specific criteria and secondary BSI
NHSN developed Table B-1 (Secondary BSI Guide) as a reference to assist users in making secondary BSI determinations. The table lists definitions that require a blood specimen with at least one matching organism to the site-specific specimen (Scenario #1) and definitions that use a blood specimen as an element to meet the site-specific definition (Scenario #2). All elements of the site-specific infection definitions must be met before a secondary BSI determination can be made. Table B-1 can be found in Chapter 4 [PDF – 1 MB] of the Device Associated Module BSI.
Secondary BSI to lower respiratory events in locations performing VAE surveillance
To determine if a positive blood culture can be attributed as a secondary bloodstream infection (BSI) related to a lower respiratory tract event, consider the following steps:
1) Does the patient meet any of the VAE definitions?
- If only the VAC or IVAC definition is met, then the positive blood culture CANNOT be secondary to the VAE (as per the VAE surveillance protocol, BSIs cannot be deemed secondary to VAC or to IVAC).
- If the PVAP definition is met, then the bloodstream infection may be attributed to the VAE (as a secondary BSI) IF the blood culture meets the VAE secondary BSI requirements as outlined in the VAE surveillance protocol: the organism isolated from blood must match an organism isolated from an appropriate respiratory tract specimen used in meeting the PVAP definition AND the blood culture must be collected during the 14-day VAE event period.
2) If the BSI cannot be attributed as a secondary BSI to VAE, then the positive blood culture can be evaluated to see if it is secondary to any of the infection sites as defined in Chapter 17 or PNEU, UTI, or SSI event protocols. If another specific site infection to which the bloodstream infection can be attributed as a secondary BSI is not identified, the BSI may need to be reported as a primary BSI/CLABSI.
See VAE FAQ #20 (https://www.cdc.gov/nhsn/faqs/faq-vae.html) for guidance on reporting secondary BSI pathogens for PVAP events.
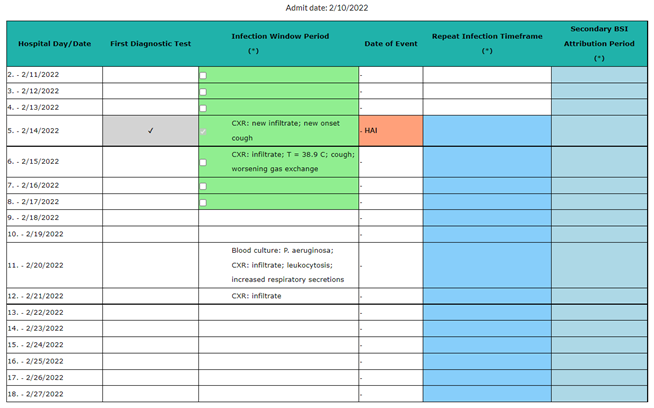
Secondary BSI assignment to a PNEU event
Yes, it is possible.
A secondary BSI cannot be attributed to PNU1. However, in this instance if a BSI is thought to be secondary to pneumonia but the blood culture collection date did not occur within the infection window period such that it could be used as an element to meet the PNU2 definition, reassess to determine if the PNU2 definition can be met within the PNEU RIT. Similarly, if PNU2 is met and there is a subsequent blood specimen with an eligible pathogen, however it does not match the pathogen used to originally meet PNU2, then reassess to determine if PNU2 can be met using the blood culture such that the date of event falls within the originally established PNEU RIT.
Note: When re-meeting PNEU within the RIT, provided that definitive imaging findings are persistent, a single definitive imaging finding with a test date that occurs in a new IWP and demonstrates evidence of persistence of prior eligible findings will satisfy the imaging requirement for all patients. New or progressive or serial imaging (for patients with underlying cardiac or pulmonary disease) does not have to be re-demonstrated. Evidence of persistence of eligible findings alone will meet the imaging requirement in the RIT. See the example and the table below.
Example
All elements necessary to satisfy the PNU1 definition occur within the PNEU infection window period 2/11 – 2/17. The PNEU date of event is 2/14. This establishes a PNEU (PNU1) RIT 2/14- 2/27 and a PNEU BSI secondary attribution period (SBAP) 2/11 – 2/27. Subsequently a blood specimen with a collection date 2/20 identifies Pseudomonas aeruginosa. The collection date is within the secondary BSI attribution period for the PNEU event; however, a secondary BSI cannot be reported for PNU1 since the blood culture is not used to satisfy the PNU1 definition and there is no site-specific culture to which the blood culture pathogen can match. If during the PNEU RIT and within a new IWP, all elements needed to meet the PNU2 definition are present to include the blood specimen and this results in a date of event within the RIT, then the BSI can be attributed as secondary to the PNEU event. The PNEU date of event remains the same (2/14), as does the originally determined RIT, secondary BSI attribution period, device association and location of attribution. If the PNU2 definition is not met as described above and if additionally, another site-specific infection for which the BSI can be attributed as a secondary BSI is not identified, then the BSI is a primary BSI/CLABSI.
Secondary BSI Assignment to a GIT 1b or IAB 2b when organisms are identified on histopathologic exam.
To deem a blood culture secondary to GIT 1b or IAB 2b when organisms are identified on histopathologic exam, you must meet the following criteria:
- The blood specimen must be an MBI Organism.
- The histopathologic evidence element and blood specimen are captured in a GIT or IAB IWP.
- The organism identified on the pathology report must match the organism in the blood specimen. See example below:
2/2: Pathology Report: “Classical budding hyphae and spores of Candida admixed with ulcer slough (intestines). 2/4: Blood culture: Candida albicans. Because the Candida in the blood culture is an MBI Organism, the histopathologic findings and blood specimen are captured in the GIT IWP, and the organism identified in the pathology report and blood specimen match, the Candida blood specimen is deemed secondary to a GIT 1b.
Capturing non-matching organisms in a positive blood specimen
If a single blood culture contains an organism that matches the site-specific specimen and an organism that does not match, the non-matching organism can be captured in the secondary bloodstream infection period (SBAP). The non-matching organism is captured only when it is in the same blood specimen with a matching organism. Additionally, the non-matching organism must be eligible for the NHSN site-specific infection. If there are subsequent blood cultures with only the non-matching organism, you must assess these blood cultures for LCBI criteria.
Example 1: A SUTI 1b criterion is cited using fever and a ≥ 100K E. coli urine culture. Additionally, a blood specimen is positive for E. coli and Enterococcus faecalis, on the next day. The E. coli and E. faecalis organisms are captured in the SBAP because the blood specimen contains the matching organism, E. coli. However, if a later blood specimen is positive for E. faecalis alone, it cannot be deemed secondary to the SUTI 1b event because the blood specimen does not contain the matching organism.
Example 2: A SUTI 1b criterion is cited using fever and a ≥ 100K E. coli urine culture. On the next day, a blood specimen is positive for E. coli and Candida albicans. The E. coli organism is captured in the SBAP because it is a matching organism. However, the Candida albicans cannot be captured in the SBAP because this organism is not eligible for SUTI criteria.
MBI-LCBI vs. Secondary BSI
MBI-LCBI criteria were implemented to enable NHSN to identify BSIs believed to be the result of a patient’s weakened immune state and the accompanying alterations of the gut. In such situations, the patient has a primary BSI, because an infection is not present at another site. However, the gut is the source of the colonizing organisms seeding the bloodstream. A BSI that is primary in nature is different from those in which the BSI is believed to be secondary to an infection at another site
To determine if a positive blood specimen is secondary to a site-specific infection, like GIT, clinical judgment should be used, and medical documentation should be reviewed to make that determination. The organisms resulted from the positive blood specimen may provide some help in determining if an infection is primary or secondary. When clinical interpretation indicates that there is an infectious process occurring in the gut, AND the patient meets one of the Chapter 17 GI infection criteria, AND the guidance in Appendix 1 Secondary BSI Guide is followed, then such BSIs should be considered secondary to another site of infection and not reported as CLABSI.
MBI-LCBI Reporting
Yes. Effective with the 2015 re-baseline, MBI-LCBIs are no longer included in the CLABSI metrics used for national reporting or in files that are shared with CMS for reimbursement determinations. A separate report has been created for MBI-LCBI tracking. However, NHSN has not changed the requirement for hospitals to include MBI-LCBIs if the locations monthly reporting plan includes CLABSI surveillance. We believe the continued collection and tracking of MBI-LCBI data is important because these infections cause significant morbidity and mortality for patients and through their identification and measurement exists the potential to identify new prevention efforts.
Distinguishing serial reportable infections from single, unresolved infection
Yes, the creation of the repeat infection timeframe (RIT) eliminated the subjectivity of trying to determine if an infection was on-going or resolved. The 14-day RIT is the period of time in which no new BSIs will be reported. The date of event (DOE) for the primary BSI is day one of the 14-day BSI RIT. Only primary BSIs create a BSI RIT. Secondary BSI’s do not create a BSI RIT, but the site-specific infection to which the BSI is secondary does create a 14-day RIT for infections of the same type. If a BSI is identified outside of the 14-day BSI RIT, a new event is identified and must be investigated as being primary or secondary in nature.
Example: If a person meets the definition for CLABSI with a positive Staphylococcus aureus culture on hospital day 12 and on hospital day 18 another blood culture is positive with an eligible pathogen, is this a new CLABSI?
In this example, if a primary BSI with a DOE on hospital day 12 is identified, and another positive blood specimen meeting LCBI criteria is identified with a DOE within the 14-day RIT, no new LCBI will be reported but any additional organisms identified will be added to the initial BSI event. The next possible DOE for a new BSI would be hospital day 26, which is day 15, the day after the 14-day RIT ended. For complete details regarding RIT, please see Chapter 2 PDF icon [PDF – 1 MB].
Note: RITs are only set when an infection criterion is met, and they are specific to the type of infection identified and do not affect reporting of other types of infections. BSIs that are secondary to another primary site of infection do not meet the NHSN LCBI criteria. A secondary BSI to a primary site of infection does not create a RIT of its own that captures all subsequent positive blood cultures. Therefore, subsequent positive blood specimens may need to be investigated to determine if they are primary BSIs and possible CLABSIs.
Device association during the BSI repeat infection timeframe (RIT)
No. Device association, the date of event, and the location of attribution are never changed from the initial determination when subsequent BSI events occur during a BSI RIT. The subsequent positive blood specimen, within the BSI RIT, is considered an extension of the initial BSI event.
Blood culture collection methods
Yes. The collection site (venipuncture site or central line drawn) of the blood specimen does not determine the eligibility of the positive blood specimen to meet CLABSI criteria. All positive blood specimens, regardless of the sites from which they were collected or the purposes for which they were drawn must be included in CLABSI surveillance. Additionally, NHSN does not require that a BSI be attributed to a particular device for reporting purposes. A BSI is determined to be central line associated (CLABSI) if an eligible central line (central line that has first been accessed and in place for more than 2 consecutive calendar days) is present on the BSI date of event (DOE) or the day before. The day of device placement is CL Day 1 or if the central line was present on admission (POA), the day of first access in an inpatient location is CL Day 1 for making a device-associated determination.
Defining "separate occasions"
The term “separate occasions” is required for laboratory-confirmed bloodstream infections (LCBIs) when only common commensals are identified in the blood (LCBI 2 or 3). The “two or more blood specimens drawn on separate occasions” element is met if there is blood collected from at least two blood draws on the same or consecutive calendar days. The requirement for at least 2 blood specimens with matching common commensals collected on separate occasions is implemented to avoid identifying a contaminated specimen as an LCBI. For the purposes of NHSN surveillance, it is expected that proper technique for specimen collection is being performed. If two positive blood cultures are assigned separate specimen numbers, processed individually, and are reported separately in the final laboratory report, the findings are considered drawn on separate occasions and eligible for use in LCBI 2/3 criteria (no exclusions).
Common commensals are a single element
The two matching common commensal blood cultures are considered a single element for use in meeting LCBI 2 or 3 criteria. If signs/symptoms occur within the infection window period (IWP) BEFORE the first collected blood specimen, the date of event (DOE) will be the date the first element (sign/symptom) used to meet LCBI 2 or 3 criteria occurred. If the sign/symptom occurs AFTER the collection date of the first blood specimen, the date of event (DOE) will be the collection date of the first positive blood specimen that occurred during the IWP.
Matching Common Commensals
Both Staphylococcus capitis and Staphylococcus auricularis are coagulase negative Staphylococci (CNS). However, they are identified to the species level, and at this level the organisms do not match. Therefore, they are not considered companion (matching) specimens. Since each organism was identified in a single blood culture, they are both considered contaminants and are disregarded for NHSN reporting purposes. LCBI 2 or 3 criterion is not met because 2 blood cultures, collected on separate occasions, with matching common commensals are not present.
If neither of the specimens had been speciated but instead were reported as coagulase-negative Staphylococcus, they would be considered companion (matching) cultures.
Central line counts for device attribution and denominator data
All central lines (permanent, temporary, implanted ports, and umbilical lines) will be treated the same for making device attribution determinations (specifically, CLABSI) and for counting denominator device days (central line days).
- Denominator data: Central lines that are present on admission should be included in device day counts beginning on the day of admission. This is consistent with the other device associated protocols (CAUTI and VAE) and is intended to simplify the collection of denominator data.
For example, a patient admitted on Jan. 1st to an inpatient location with a central line in place that is first accessed on Jan. 4th, has a BSI DOE on Jan 6th. A total of 6 central line days will be entered for the denominator device day count.
Device-attribution (CLABSI event): An eligible central line must be present to make a device-associated determination (CLABSI). An eligible central line is a device that has first been accessed in an inpatient location and has been in place for more than 2 consecutive calendar days. “Access” is defined as line placement, or use of the line for infusion, withdrawal of blood or hemodynamic monitoring. Once a line becomes an eligible central line (CL Day 3 after access), it continues to be eligible for a CLABSI event until the day after the line is removed from the body or the day after patient is discharged, whichever comes first. For example, a patient admitted on Jan. 1st to an inpatient location with a central line in place that is first accessed on Jan. 4th, has a BSI DOE on Jan 6th. Because the line was first accessed in an inpatient location, and it has been in place for more than 2 consecutive calendar days on Jan. 6th, it is eligible for a CLABSI event. If the line is never accessed, it never becomes eligible for a CLABSI event but is included in the denominator device day counts.
Permanent Catheter Example(s):
Example 1:
A patient is seen in the ED with a permanent catheter (for example port) in place on Dec 31st. The permanent catheter is accessed in the ED for IV fluids. Patient is admitted on Jan. 1st to an inpatient location with IV fluids infusing into the port. Jan 1st is central line day 1 for making a CLABSI determination since fluids are infusing into the port when the patient locates to the inpatient location. There is a BSI DOE on Jan 6th. Because the line was first accessed in an inpatient location on Jan 1st, and it has been in place for more than 2 consecutive calendar days on Jan. 6th, it is eligible for a CLABSI event.
Example 2:
A patient is seen in the ED with a permanent catheter (for example port) in place on Dec 31st. The permanent catheter is accessed in the ED for IV fluids. Patient is admitted on Jan. 1st to an inpatient location, and there are no IV fluids infusing into port when the patient arrives to the inpatient location. The permanent catheter is accessed on Jan 5th, and there is a BSI DOE on Jan 6th. The line is first accessed in an inpatient location on Jan 5th when the permanent catheter is accessed in the inpatient location but has not been accessed for more than 2 consecutive calendar days on Jan. 6th. Therefore, the line is not eligible for a CLABSI event.
If the line is never accessed, it never becomes eligible for a CLABSI event but is included in the denominator device day counts.
Note: If a patient has another central line in place that is accessed, device attribution (eligible for a CLABSI event) begins on CL Day 3 after access.
Removal and reinsertion of a central line
If a central line is present for any part of a calendar day, then that day contributes to the minimum day’s requirement for the CLABSI. If a full calendar day passes without a central line being present, then the device day count for making a CLABSI determination starts over once a new CL is inserted. Please see Table 3 of the NHSN BSI Protocol [PDF – 900 KB] for examples.
Midline catheter
Midline catheters by description are not intended to terminate in one of the great vessels or near the heart which is a part of the definition of a central line. However, the actual location of the catheter tip is the determining factor, and the chest x-ray obtained to verify line placement should indicate the location of the tip. Also, consider the line’s intended use. To qualify as a central line, it must be used for infusion, withdrawal of blood, or hemodynamic monitoring. Keep in mind both elements of NHSNs central line definition are required. If both requirements are not met, the device is not a central line.
Catheter tips
Catheter tip cultures are not used for NHSN CLABSI surveillance for several reasons. Catheter tip cultures have been shown to have higher rates of contamination than blood cultures. Furthermore, not all laboratories are able to perform quantified catheter tip cultures. Catheter tips are a part of other types of non-NHSN surveillance such as catheter-related BSI (CRBSI) which is generally considered a clinical definition, used when diagnosing and treating patients.
The Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011 address CRBSI and may be helpful when addressing a physician’s questions.
Multiple central lines
NHSN does not require a CLABSI to be attributed to a specific central line. Instead, you will simply be required to document whether an eligible central line was present on the BSI date of event or the day before. An eligible central line is a device that has first been accessed in an inpatient location and has been in place for more than 2 consecutive calendar days.
Only 1 central line day is counted per patient-per calendar day regardless of how many lines the patient may have in place at the same time. If a patient in a SCA/Oncology unit has both a temporary and a permanent line, only report the temporary line because it has a higher risk of infection.
Pus or purulence at the vascular access site
- Both a central line and another vascular access device in place.
- Pus at the other vascular access device site.
- Matching organisms from the vascular access site and blood specimen.
Please see Note: #d, under the Comments and Reporting Instructions found in Chapter 4 BSI [PDF – 900 KB] protocol for guidance. This guidance states:
Occasionally, a patient with both a central line and another vascular access device will have pus at the other access site. If there is pus at the site of one of the following vascular access devices and a specimen collected from that site has at least one matching organism to an organism identified in blood during the BSI infection window period, report such events, marking the “pus at the vascular access site” field as “Yes.” In this situation, enter “Yes” for the field “Central Line.” However, you should include the patient’s central line days in the summary denominator count. Vascular access devices included in this exception are limited to:
- Arterial catheters, unless in the pulmonary artery, aorta, or umbilical artery
- Arteriovenous fistulae
- Arteriovenous graft
- Atrial catheters (also known as transthoracic intra-cardiac catheters, those catheters inserted directly into the right or left atrium via the heart wall)
- Hemodialysis reliable outflow (HERO) dialysis catheters
- Intra-aortic balloon pump (IABP) devices
- Non-accessed central line (not accessed nor inserted during the hospitalization)
- Peripheral IV
- Midlines unless the central line definition is met
Intra-aortic balloon pumps (IABP)
IABPs are not considered central lines since they are generally not used for infusion, blood withdrawal, or hemodynamic monitoring. Do not include IABP device days in the central line day count.
IABPs are not considered ventricular assist devices. The bloodstream infection risks associated with IABP devices are different from an NHSN surveillance perspective.
Femoral arterial lines
The femoral artery is not among the list of great vessels defined for CLABSI surveillance in NHSN, therefore, a catheter in this vessel is not considered a central line. Do not include femoral artery catheter days in the central line day count.
Dialysis patients
In both situations, the CLABSI must be attributed to the inpatient location where the patient is housed. The dialysis unit is defined as a non-bedded inpatient unit that provides services to inpatients. CLABSI surveillance, for NHSN reporting purposes, cannot be performed in this location because there can be no patient or central line day counts. Likewise, dialysis performed in the patient’s room by dialysis staff cannot be attributed to the dialysis unit. The BSI event form contains the following required field “Any hemodialysis catheter present?”. The data in this field can be used internally by facilities to identify and track potential issues related to dialysis care. Data in the “Any hemodialysis catheter present?” field are not used to exclude events from the CLABSI SIR.
Contracted staff
Facilities are responsible for all patient care provided in the facility by employed and contracted staff alike. Therefore, all CLABSIs identified in dialysis patients are attributed to the in-patient unit where the patient is physically located.
Patient suspected of injecting into vascular catheter
A positive blood specimen meeting an LCBI criteria, that is accompanied by documentation, during the infection window period (IWP), of observed or suspected patient injection into vascular access lines during the inpatient admission, will be excluded from CLABSI surveillance. This exclusion is very specific to INJECTION into the line (tampering with, manipulating, etc. do not meet the intent of the exclusion). For example, “pulling on the line, picking at the dressing, removing the caps, and biting” are just a few examples of line manipulation that do not meet the requirements of this exception. NHSN considers these actions different from purposeful injection into a vascular line without regard for proper line use and/or management and places the patient’s health and safety at risk.
- These events are still considered LCBIs but are not associated with the central line and are not CLABSIs for NHSN reporting purposes. When reporting the BSI to NHSN, answer “Yes” to the central line and self-injection fields.
- A BSI repeat infection timeframe (RIT) is created during which no new BSI events will be reported, but any additional organisms identified are added to the initial event. Positive blood specimens collected outside of the BSI RIT must be investigated as primary or secondary.
Note:
- Injection, in this exclusion, is limited to patient injection only and does not include injection performed by other healthcare providers whether employed or contracted, family members, or visitors.
Vital Signs (hypotension, apnea, bradycardia)
NHSN does not define a specific value for these vital signs. Instead, each facility should use vital sign parameters as stated in their policies and procedures for clinical documentation. The presence of any of these vital signs before the IWP does not exclude the event from meeting LCBI 2 or 3 criteria. An LCBI 2 or 3 criterion may be assigned if an eligible vital sign occurs during the BSI IWP.
Reporting Locations
Information on locations that are included in reporting can be found in the Operational Guidance for Acute Care Hospitals to Report Central Line-Associated Bloodstream Infection (CLABSI) [PDF – 169 KB]. The Centers for Medicare and Medicaid Services are the ultimate source for identifying reporting required as part of their programs.
Submitting a BSI case review to NHSN
For NHSN to assist with a primary or secondary BSI case determination please send the following information to the NHSN Helpdesk:
If investigating a positive blood culture:
- Admission date
- Central line insertion date
- Central line discontinuation date if applicable
- Date(s) and results of any positive blood cultures
- All organisms identified in the blood culture(s)
- Signs/symptoms and associated dates if evaluating LCBI-2/3 criteria
- Date of first access in an inpatient location (if patient is admitted with a central line in place)
- MBI LCBI risk factors (if evaluating MBI LCBI criteria)
If evaluating for a secondary BSI:
- Site specific infection under consideration (for example Chapter 17 infections, SSI, UTI, PNEU)
- Supporting documentation (for example any positive blood cultures, imaging results, or sign/symptoms and associated dates if applicable)
- Date(s) and results of any positive blood cultures
- All organisms identified in the blood culture(s) (include information on whether the organisms are in the same blood culture or two separate blood cultures)
- Any information on recent NHSN surgical procedures (including the operative report and any imaging performed)
Central Line Associated Bloodstream Infection (CLABSI) Exclusions
Yes, these events should be reported to NHSN. Although the events are reported, they are NOT considered central line associated and DO NOT contribute to the CLABSI SIR measure. Exclusions include:
- Extracorporeal life support (ECLS [for example ECMO])
- Epidermolysis bullosa (EB)
- Munchausen syndrome by proxy (MSBP)
- Pus at the vascular access site
- Self-injection
- Total Artificial Heart (TAH)
- Ventricular assist device (VAD)
MBI RIT Exception
An MBI-LCBI designation will not change to an LCBI event if the two criteria below are met:
- The positive non-MBI blood culture is collected during an MBI-LCBI BSI RIT.
- The positive non-MBI blood culture can be deemed secondary to an NHSN site-specific infection.
Please see Example 2b in Chapter 2 [PDF – 909 KB].
Non-culture Based Testing Method (NCT)
Yes, NCT methods are eligible for use to meet LCBI-1 criterion. If an organism is identified to the genus or species level by non-culture based microbiologic testing methods.
Note: If blood is collected for culture within 2 days before, or 1 day after the NCT, disregard the result of the NCT and use only the result of the CULTURE to make an LCBI surveillance determination. If no blood is collected for culture within this time period, use the result of the NCT for LCBI surveillance determination
Yes, NCT methods are eligible for use to meet LCBI-1 criterion. If an organism is identified to the genus or species level by non-culture based microbiologic testing methods.
Note: If blood is collected for culture within 2 days before, or 1 day after the NCT, disregard the result of the NCT and use only the result of the CULTURE to make an LCBI surveillance determination. If no blood is collected for culture within this time period, use the result of the NCT for LCBI surveillance determination
AND
Organism(s) identified in blood is not related to an infection at another site (See LCBI-1 Criterion in the BSI Chapter [PDF – 1 MB]).
Note: If blood is collected for culture within 2 days before, or 1 day after the NCT, disregard the result of the NCT and use only the result of the CULTURE to make an LCBI surveillance determination. If no blood is collected for culture within this time period, use the result of the NCT for LCBI surveillance determination