Antimicrobial-Resistant Aspergillus
Azole-Resistant Aspergillus Fumigatus
(A. fumigatus)
Antimicrobial resistance happens when germs like bacteria and fungi develop the ability to defeat the drugs designed to kill them. That means the germs are not killed and continue to grow. Antimicrobial resistance is emerging in one type of Aspergillus called Aspergillus fumigatus (A. fumigatus), a common mold in the environment and the leading cause of invasive mold infections in people.1, 2 In CDC’s 2019 Antibiotic Resistance Threats Report, azole-resistant A. fumigatus is on the Watch List, a category of bacteria and fungi that have caused few resistant infections in the United States to date but have the potential to rapidly spread. This public health threat requires attention in the healthcare and the environmental sectors.
Azole-Resistant Aspergillosis
A. fumigatus can cause an invasive life-threatening infection, called aspergillosis, in people who have weakened immune systems, have underlying diseases, or have had transplants.3 Patients with severe cases of respiratory infections (like influenza or COVID-19) have also developed aspergillosis. Triazole antifungal drugs, commonly called azoles, are the primary treatment for aspergillosis. Azole-resistant A. fumigatus infections are difficult to treat, and these patients are up to 33% more likely to die than patients with infections that can be treated with azoles.3
Emergence Globally and Within the United States
At medical centers in some parts of the world, an estimated 19% of A. fumigatus infections are resistant to azole antifungals.3 In a large U.S. study, antimicrobial resistance was identified in up to 7% of Aspergillus specimens from patients with stem cell and organ transplants.5-7
The true burden of azole-resistant aspergillosis in the United States is unknown and a few factors make it difficult to estimate:
- Aspergillosis is one of the leading missed diagnoses in intensive care units.
- Until recently, clinicians have had limited access to antifungal susceptibility testing.
Azole-Resistance Testing: Information for Healthcare and Clinical Labs
Select regional labs in the AR Lab Network perform screening to monitor and track the emergence of A. fumigatus azole resistance in the United States. Testing is available to all states.
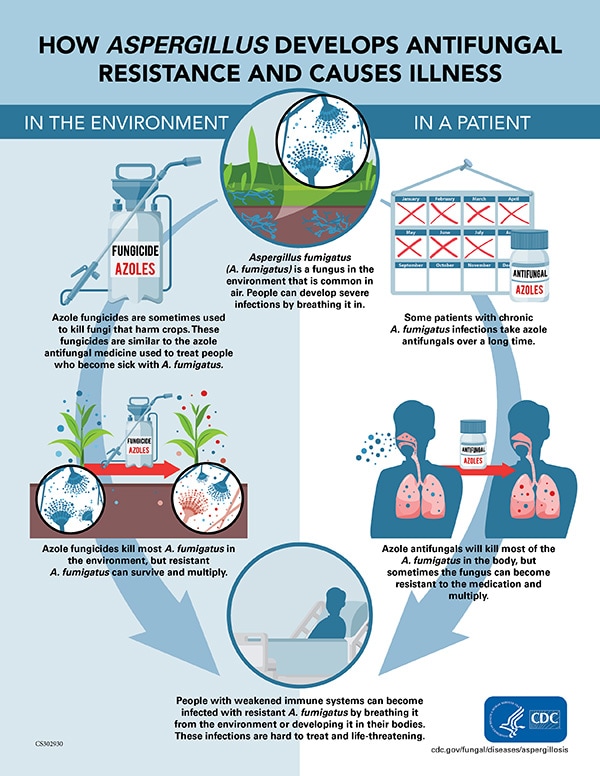
Azole Resistance Develops in the Body and in the Environment
We know of two ways that A. fumigatus can develop resistance to azoles.
- Inside the body: Strains of A. fumigatus in people who take azole antifungals for a long period of time can become resistant, survive treatment, and continue to cause infection.
- Outside the body: Strains of A. fumigatus on decaying plants in the environment can be exposed to azole compounds used as fungicides that are chemically similar to azole antifungal medications. These strains can develop resistance to azoles. 8-11
Azole Fungicide Use
Azole fungicides are used throughout the world to treat plant infections, prevent crop loss, and increase production. Patients with azole-resistant aspergillosis have been infected with A. fumigatus strains with the same resistance gene markers (TR34/L98H and TR46/Y121F/T289A) as resistant strains found in the environment associated with agricultural azole fungicide use.3, 14 Resistant Aspergillus infections are also found in people who have not taken azole antifungals, further suggesting that the resistance is partially driven by environmental sources.12 While this is becoming common in Europe, so far only a small number of cases have been identified in the United States.
Estimates from the United States Geological Survey show that agricultural azole fungicide use quadrupled (434%) in the United States from 2013 to 2016.13 A direct association between the quantity of U.S. agricultural azole fungicide use and human infections has not been established.
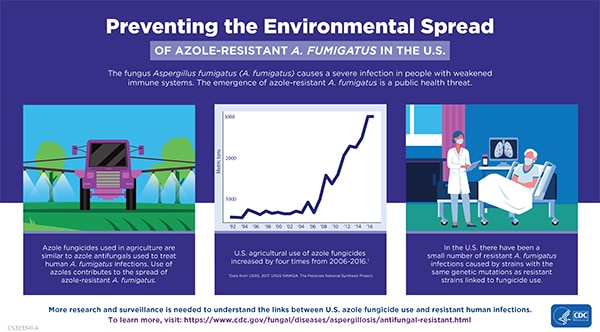
CDC is working with partners to assess the potential impact of increased fungicide use in the United States on the development of azole resistance and establishing initiatives to help protect public health while maintaining healthy and stable crop production.
Preventing Azole Resistance
Any use of antifungals can contribute to the development of antimicrobial resistance. Ensuring appropriate use of azoles in human medicine, agriculture, and industry will be essential to curbing the spread of antimicrobial resistance. In clinical settings, healthcare providers can adopt antifungal stewardship practices and order susceptibility testing when aspergillosis is suspected. In serious cases where testing is not readily available, healthcare providers should consult their state or local health department. The agricultural community, phytologists, and federal partners should work together to consider alternative ways to protect and treat crops. Integrated pest management (IPM) is one strategy that has been recognized internationally by the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) to successfully reduce the need for pesticide use and improve food quality and crop yields.
What CDC Is Doing
CDC is working with the Environmental Protection Agency, the U.S. Department of Agriculture, and other agencies to address issues surrounding the environment and antimicrobial resistance.
CDC is leading and supporting several projects through the AR Solutions Initiative including:
- Expanding resistance testing for A. fumigatus clinical isolates through the AR Lab Network.
- Supporting the University of California (UCLA) in a two-year project establishing a facility-level surveillance system for influenza-associated Aspergillus infections.
- Working with the Mycoses Study Group and several U.S. medical centers to identify COVID-19–associated pulmonary aspergillosis and other secondary fungal infections.
- Supporting a University of Georgia project testing environmental samples from agricultural sites in different regions of the United States to better assess the presence of triazole-resistant A. fumigatus in the environment.
- Berkow EL, Nunnally NS, Bandea A, Kuykendall R, Beer K, Lockart SR. Detection of TR34/L98H CYP51A mutation through passive surveillance for azole-resistant Aspergillus fumigatus in the United States from 2015 to 2017. Antimicrob Agents Chemother 2018;62:e02240–17.
- Beer KD, Farnon EC, Jain S, Jamerson C, Lineberger S, Miller J, et al. Multidrug-resistant Aspergillus fumigatus carrying mutations linked to environmental fungicide exposure — three states, 2010–2017. MMWR Morb Mortal Wkly Rep 201;67:1064–7.
- Lestrade PP, Bentvelsen RG, Schauwvlieghe AFAD, Sch 452 alekamp S, van der Velden WJFM, Kuiper EJ, et al. 2019. Voriconazole Resistance and Mortality in Invasive Aspergillosis: A Multicenter Retrospective Cohort Study. Clin Infect Dis 68:1463–1471; doi:10.1093/cid/ciy859.
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012;4:165rv13.
- Baddley JW, Marr KA, Andes DR, Walsh TJ, Kaufman CA, Kontoyiannis DP, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J Clin Microbiol 2009;47:3271–5.
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010;50:1091–100.
- Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010;50:1101–11.
- Brauer VS, Rezende CP, Pessoni AM, De Paula RG, Rangappa KS, Nayaka SC, et al. Antifungal agents in agriculture: friends and foes of public health. Biomolecules 2019;9:521.
- Hurst SF, Berkow EL, Stevenson KL, Litvintseva AP, Lockhart SR. Isolation of azole-resistant Aspergillus fumigatus from the environment in the south-eastern USA. J Antimicrob Chemother 2017;72:2443–6.
- Snelders E, Camps SMT, Karawajczyk A, Schaftenaar G, Kema GHJ, van der Lee HA, et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 2012;7:e31801.
- Verweij PE, Kema GH, Zwaan B, Melchers WJ. Triazole fungicides and the selection of resistance to medical triazoles in the opportunistic mould Aspergillus fumigatus. Pest Manag Sci 2013;69:165–70.
- Snelders E, Huis in ’t Veld RAG, Rijs AJMM, Kema GHJ, Melchers WJG, Verweij PE. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 2009;75:4053–7.
- Toda Mitsuru, Beer Karlyn D., Kuivila Kathryn M., Chiller Tom M., Jackson Brendan R. Trends in Agricultural Triazole Fungicide Use in the United States, 1992–2016 and Possible Implications for Antifungal-Resistant Fungi in Human Disease. Environ Health Perspect 129:055001; doi:10.1289/EHP7484.
- Langfeldt A, Gold JAW, Chiller T. Emerging Fungal Infections: from the Fields to the Clinic, Resistant Aspergillus fumigatus and Dermatophyte Species: a One Health Perspective on an Urgent Public Health Problem. Curr Clin Micro Rpt (2022)