One Health and Fungal Diseases
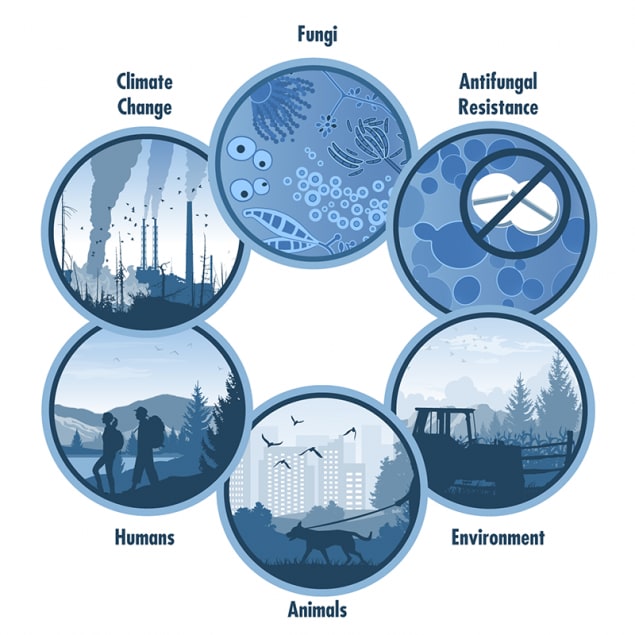
One Health is an approach that recognizes that the health of people is closely connected to the health of animals and our shared environment. One Health involves experts in various fields working together at the local, regional, national, and global levels to improve health. Disease spread by fungi are a One Health issue—fungi that cause human diseases live in the environment and some can spread between animals and people.
Fungi are everywhere. There are millions of fungal species, but only a few hundred of them are known to make people sick. Some fungal diseases, such as ringworm, are zoonotic—meaning that the disease can spread from animals and people. Other fungal diseases, like histoplasmosis, can’t spread from animals and people, but can infect both animals and humans who are exposed to fungi in the environment. Exposure to fungi can also cause other health problems, like asthma and allergies. Natural disasters like flooding can cause fungi to grow quickly. Changes in the environment caused by urban growth and intensive farming practices can also affect fungi and their spread. A One Health approach is important for the prevention and control of fungi that can spread through the environment and between animals and people.
We are only beginning to understand how the interconnections between people, animals, and the environment affect fungal diseases. The following examples show these connections:
- Ringworm is a common fungal skin infection that affects humans and animals. People can get ringworm from contact with infected animals or the environment.
- Over half of outpatient doctor visits for fungal diseases are for ringworm.1
- The use of antifungal drugs in the environment may be causing antifungal resistance. Antifungal resistance happens when fungi develop the ability to defeat the drugs meant to kill them. Resistant fungi can make infections harder to treat in both people and animals.
- Triazole antifungal drugs are the main treatment for Aspergillus infections in animals and people. Triazole fungicide use in plant agriculture may lead to triazole-resistant Aspergillus infections, which are difficult to treat.2
- Because of climate change, the environments where disease-causing fungi might live are expanding.
- In the United States, environmental fungal diseases that may become more common because of climate change include blastomycosis,3–6 Valley Fever,7–10 histoplasmosis,11 Cryptococcus gattii infection,11,12 mold-related illnesses,13 and Candida auris.14,15
- Climate change is increasing the frequency, intensity, and duration of extreme weather events, such as drought and wildfires. These events can encourage the production and spread of fungal spores that cause disease.16
- New zoonotic disease-causing fungi, such as Sporothrix brasiliensis, are becoming more common.
- Sporotrichosis, caused by Sporothrix brasiliensis, is a zoonotic disease that can spread among cats and from cats to humans. This disease is rapidly expanding to new regions in South America.17
- Fungi can impact human and animal health in other ways.
- Toxins produced by fungi associated with agriculture can affect human and animal health when consumed.
- Contamination of food crops with aflatoxins, produced by Aspergillus, has led to pet aflatoxin poisonings and pet food recalls in the United States. Aflatoxins can also cause sickness in humans.18
- Eating poisonous mushrooms can serious illness and death in humans and animals.19,20
- Damp and moldy environments can lead to allergy symptoms in people and animals. During and after severe weather events, flooding can lead to excess moisture and mold growth. Preventing excess moisture in your home can help prevent mold.
- Toxins produced by fungi associated with agriculture can affect human and animal health when consumed.
- Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clinical Infectious Diseases. 2019;68(11):1791-
- Toda M, Beer KD, Kuivila KM, Chiller TM, Jackson BR. Trends in Agricultural Triazole Fungicide Use in the United States, 1992–2016 and Possible Implications for Antifungal-Resistant Fungi in Human Disease. Environmental Health Perspectives. 2021;129(5):055001.
- Klein BS, Vergeront JM, Disalvo AF, Kaufman L, Davis JP. Two Outbreaks of Blastomycosis Along Rivers in Wisconsin: Isolation of Blastomyces Dermatitidis From Riverbank Soil and Evidence of Its Transmission Along Waterways. American Review of Respiratory Disease. 1987;136(6):1333-8.
- Klein BS, Vergeront JM, Weeks RJ, Kumar UN, Mathai G, Varkey B, et al. Isolation of Blastomyces Dermatitidis in Soil Associated With a Large Outbreak of Blastomycosis in Wisconsin. New England Journal of Medicine. 1986;314(9):529-34.
- Pfister JR, Archer JR, Hersil S, Boers T, Reed KD, Meece JK, et al. Non-Rural Point Source Blastomycosis Outbreak Near a Yard Waste Collection Site. Clinical Medicine & Research. 2011;9(2):57-65.
- Proctor ME, Klein BS, Jones JM, Davis JP. Cluster Of Pulmonary Blastomycosis in a Rural Community: Evidence for Multiple High-Risk Environmental Foci Following a Sustained Period Of Diminished Precipitation. Mycopathologia. 2002;153(3):113-20.
- Pappagianis D. Marked Increase in Cases of Coccidioidomycosis in California: 1991, 1992, and 1993. Clinical Infectious Diseases. 1994;19(Supplement_1):S14-S8.
- Gorris M, Cat L, Zender C, Treseder K, Randerson J. Coccidioidomycosis Dynamics in Relation to Climate in The Southwestern United States. GeoHealth. 2018;2(1):6-24.
- Tong DQ, Wang JX, Gill TE, Lei H, Wang B. Intensified Dust Storm Activity and Valley Fever Infection In The Southwestern United States. Geophysical Research Letters. 2017;44(9):4304-12.
- Weaver EA, Kolivras KN. Investigating The Relationship Between Climate and Valley Fever (Coccidioidomycosis). EcoHealth. 2018;15(4):840-52.
- Hanf M, Adenis A, Carme B, Couppié P, Nacher M. Disseminated Histoplasmosis Seasonal Incidence Variations: a Supplementary Argument For Recent Infection? J AIDS Clinic Res. 2012;3(8):1000175.
- Datta K, Bartlett KH, Marr KA. Cryptococcus gattii: Emergence in Western North America: Exploitation of a Novel Ecological Niche. Interdisciplinary Perspectives on Infectious Diseases. 2009.
- Benedict K, Park BJ. Invasive Fungal Infections After Natural Disasters. Emerging Infectious Diseases. 2014;20(3):349-355
- Casadevail A, Kontoyiannis DP, Robert V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio. 2019.
- Jackson BR, Chow N, Forsberg K, Litvintseva AP, Lockhart SR, Welsh R, et al. On the Origins of a Species: What Might Explain the Rise of Candida auris? Journal of Fungi. 2019; 5(3):58.
- Kobziar LN, Thompson GR. Wildfire Smoke, a Potential Infectious Agent. 2020; 370 (6523): 1408-1410.
- Gremião IDF, Oliveira MME, de Miranda LHM, Freitas DFS, Pereira SA. Geographic Expansion of Sporotrichosis, Brazil. Emerging Infectious Diseases. 2020;26(3):621.
- Stenske KA, Smith JR, Newman SJ, Newman LB, Kirk CA. Aflatoxicosis in Dogs and Dealing with Suspected Contaminated Commercial Foods. Journal of the American Veterinary Medical Association. 2006;228(11):1686-91.
- Gold JA, Kiernan E, Yeh M, Jackson BR, Benedict K. Health Care Utilization and Outcomes Associated with Accidental Poisonous Mushroom Ingestions — United States, 2016–2018. MMWR Morb Mortal Wkly Rep 2021;70:337-341
- Kaae JA, Poppenga RH, Hill AE. Physical Examination, Serum Biochemical, and Coagulation Abnormalities, Treatments, and Outcomes for Dogs with Toxicosis from Α-Amanitin–Containing Mushrooms: 59 Cases (2006–2019). Journal of the American Veterinary Medical Association. 2020; 258(5), 502-509.