TB NOTES

TB Notes 3, 2021
Notes from the Director

Dear Colleague,
CDC recently released the 2020 edition of Reported Tuberculosis in the United States. The report describes information on TB disease reported to the CDC since 1993, with an emphasis on TB disease cases counted by the reporting area in 2020, and the effects of the COVID-19 pandemic on TB disease surveillance. The 2020 data reveal a substantial decline in the number of reported cases of TB disease in the United States.

The COVID-19 pandemic has probably affected TB incidence in the United States in several ways, including a combination of TB underdiagnosis and a true reduction in incidence. CDC is conducting additional analyses to better understand how the 2020 TB disease data compare with previous years. I want to thank all state and local health departments throughout the United States whose staff collected and reported the data used in this publication.
In September 2021, Division of Tuberculosis Elimination (DTBE) launched the Tuberculosis Epidemiologic Studies Consortium III (TBESC III), with the purpose of gaining a better understanding of how latent TB infection screening, testing, and treatment is offered to patients at risk for latent TB infection and TB disease in primary care settings, as well as the impact of interventions to increase the number of eligible patients who complete latent TB infection treatment.
CDC continues to work closely with our partners at state and local health departments and the U.S. Food and Drug Administration (FDA) in response to a tuberculosis (TB) cluster among surgery patients in several states. CDC, state and local health departments, TB Centers of Excellence, and healthcare providers are collaborating to provide expert clinical consultation for patients being treated for TB disease.
In addition, DTBE is working closely with our colleagues at CDC’s Division of Global Migration and Quarantine, as well as state and local health departments to support Operation Allies Welcome. DTBE is sharing available news and updates with TB programs, providing medical consultation through the TB Centers of Excellence, and has translated the TB Q&A booklet and a TB information fact sheet into Dari and Pashto.
As health departments and healthcare facilities across the United States continue to shift staff roles, responsibilities, and resources to support the response to COVID-19, TB programs are carrying on the important work of TB prevention and control.
DTBE is also maintaining our support to CDC’s COVID-19 response. As of October 5, 2021, a total of 121 DTBE staff members (76% of DTBE staff) have participated in a cumulative of 307 deployments supporting CDC’s COVID-19, border and Hurricane Ida response efforts. A total of 73 DTBE staff members have participated in multiple deployments. Thank you to everyone who has responded.
These are just a few highlights from our work over the past quarter. I invite you to read further for additional news, resources, and success stories from across DTBE.
Thank you for your continued efforts towards our goal of TB elimination.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV, Viral Hepatitis, STD, and TB Prevention
The Tuberculosis Trials Consortium Virtual Meeting was held from October 26-28, 2021.
Dr. Andrew Vernon has accepted a new position as Associate Director for Science within the Epidemiology Task Force in the CDC COVID-19 Response. He began serving in his new position on October 10. During his tenure as Chief, Clinical Research Branch (CRB), DTBE, Dr. Vernon oversaw major advancements in TB research through seminal clinical trials, including TBTC Studies 26, 31 and 33, which led to shorter and improved treatment regimens for TB disease and LTBI. These advancements are among the most important in TB patient care in the last several decades. DTBE thanks Dr. Vernon for his excellent service in the cause of TB elimination, congratulates him on his selection, and wishes him success in this new endeavor. Dr. Vernon was also selected as the NCHHSTP Nominee for the Charles C. Shepard Lifetime Achievement Award.
Congratulations to CRB’s Tuberculosis Trials Consortium Study 31 Team, Annual CDC/ATSDR Honor Award Recipients!
The CDC and Agency for Toxic Substances and Disease Registry (ATSDR) Honor Awards highlight notable and significant achievements over the past calendar year, with some selected awards, presented by the CDC Foundation, given in recognition of career contributions while at the agency. Encompassing categories across scientific, program, management and operations sectors, the awards, which began in 1952, seek to honor employees for improving agency performance and helping ensure our efforts are not only effective, but also as far-reaching as possible.
Congratulations to the Tuberculosis Trials Consortium (TBTC) Study 31 Team on their award for Excellence in Epidemiology (International). The TBTBC Study 31 Team received the award for their exceptional epidemiologic performance in exemplary and innovative approaches to team building and problem solving.
The landmark Study 31/ A5349 was led by TBTC with collaboration from the AIDS Clinical Trials Group (ACTG) funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. Study 31/A5349- an international, randomized, controlled, open label, phase 3 non-inferiority clinical trial– demonstrated that a shorter four-month daily treatment regimen with high-dose (“optimized”) rifapentine and moxifloxacin is as effective as (non-inferior to) the standard six-month daily regimen in curing drug-susceptible TB disease. The results of this study will help inform future TB disease treatment guidelines and innovations for designing future clinical trials.
TBTC Study 31 had more than 25 investigators on the protocol team from more than 15 institutions. CDC members included Clinical Research Branch staff (Kia Bryant, Wendy Carr, Crystal Carter, Melissa Fagley, Kimberly Hedges, Connie Henderson, Amanda Hott, Carla Jeffries, Ekaterina Kurbatova, Joan Mangan, Lakshmi Peddareddy, Jessica Ricaldi, Claire Sadowski, Nigel Scott, Erin Sizemore, Andrew Vernon, William Whitworth, and Yan Yuan), as well as DTBE Laboratory Branch staff (Scott Burns, Lauren Cowan, Mindy Dunn, Jamie Posey, and Melisa Willby).
Read the press release, published study in the New England Journal of Medicine, and learn more about the Tuberculosis Trials Consortium.
Submitted by Carla Jeffries, JD, MPH
Updated resource: Questions & Answers About Tuberculosis
CDC’s updated Questions & Answers About Tuberculosis pamphlet is now available in Dari, Pashto, Spanish, Tagalog, Vietnamese, and English.
Since 1994, the Questions & Answers About TB pamphlet has been a key patient education resource for answering common questions about tuberculosis. The 2021 edition has a new look with updated, basic information on TB infection and TB disease. Key audiences for this pamphlet include people with or at risk for TB; people who may have been exposed to someone with TB; people who provide services for those at risk for TB, such as correctional officers, homeless shelter workers, and emergency responders; and people who want to learn more about tuberculosis.
This publication is available online and can be downloaded from the DTBE Website. Print copies are also available free of charge from CDC-Info on Demand Publications. For information on how to order print copies, please see the Publication Ordering Instructions.
In addition, a fact sheet, Tuberculosis: General Information, is also available on the CDC website in Dari [PDF – 11MB], Pashto [PDF – 11MB], and English [PDF – 10MB].
Submitted by Peri Hopkins, MPH, Health Education Specialist

New Look for the CDC Latent TB Infection Resource Hub
The newly redesigned CDC Latent TB Infection Online Resource Hub hosts a repository of resources and materials. On the hub, you will find resources for healthcare providers, resources for patients, as well as social media graphics and infographics.
These materials have recently been reorganized to help users easily find testing and treatment resources, as well as provider and patient education materials. The webpage is updated regularly, and a “Featured Resources” section highlights new resources, as well as those that may be of interest to the TB community.
Submitted by Kevin Crooks, MPH, Health Communications Specialist
Developing and Implementing a Remediation Plan based on Program Evaluation: A New Web Tool Available for TB Programs
The Program Evaluation and Health Economics Team of CDC’s Division of Tuberculosis Elimination is delighted to introduce a new program evaluation tool focused on remediation and program improvement. Designing and implementing a remediation plan can help TB programs take their program evaluation results to the next step in continuous quality improvement. This tool describes how TB programs can use program evaluation as an opportunity to improve their program through planning and implementation of remediation strategies based on program evaluation findings. This tool is available on the CDC website at https://www.cdc.gov/tb/programs/evaluation/remediation-plan.htm.
Submitted by Maureen Kolasa, RN, MPH
Partnering to Provide Better TB Care Yields Better Outcomes
Background: Arlington County (Virginia) Public Health Division (ACPHD) has a history of leveraging partnerships to better care for our community. When a community-based clinic, Arlington Free Clinic, identified a potential TB case, they immediately contacted us for follow-up. Early in the process it became apparent that ACPHD would need assistance providing services for the patient. Resource coordination had to go beyond the health department, the County, and even the Virginia Department of Health’s (VDH) TB program. We will describe how partnerships and resource coordination led to the successful treatment of our patient with pre-extensively drug resistant TB and protected our community from TB transmission.
Overcoming Challenges: Multidrug-resistant and extensively drug-resistant TB cases are rare in our County, which presented challenges to coordinating services. Managing this complex case required providing medical treatment, engaging the patient in care, and supporting the patient’s mental wellbeing—all while protecting our community from infection. This would not have been possible without careful coordination among partners.
Providing medical treatment: Despite being treated twice before for TB in a country outside the United States, the patient’s chest x-ray and symptoms were consistent with TB disease. We anticipated that the patient would have drug-resistant TB and require surgery to remove the remains of an affected lung. Drug-susceptibility testing performed by CDC and National Jewish Health classified the patient’s TB as pre-extensively drug resistant—resistant to all first line drugs and multiple critical second-line drugs. The Virginia State Pharmacy provided appropriate pharmaceuticals.
The patient initially weighed less than 90lbs and needed significant support to gain weight for surgery. A nutritionist from ACPHD’s Women, Infants and Children program assessed the patient’s diet, while other staff purchased groceries based on the patient’s preferences. This personalized the patient’s care with nutritious and culturally appropriate meals.
The patient had previously undergone surgery for pulmonary TB outside of the United States. The prospect of further surgery necessitated medical expertise beyond our typical capacity. A team of experts was assembled, including VDH and the University of Virginia (UVA) Medical Center. One of UVA’s surgeons was trained in Russia and familiar with procedures not commonly performed in the United States. This competence contributed to successful removal of residual lung tissue that had perpetuated her infection.
Engaging the patient in care and supporting her mental wellbeing: The complexity of the patient’s care called for interpretation and translation services. We involved a nurse from ACPHD’s School Health program who fluently spoke the patient’s primary language. She explained medications, accompanied the patient to clinical visits, and provided social support.
Along with our partners, we supported the patient’s mental and emotional wellbeing. We provided computer tablets to the patient and patient’s family to video chat during the isolation period, we provided a TV to watch the Olympics, and we brought treats for special occasions. Arlington Free Clinic provided mental health care to reduce the patient’s stress and depression.
Protecting the community: While the patient was infectious, months of isolation from the patient’s household and the community were required. County partners helped identify, furnish, and pay for an apartment with an outside entrance and separate HVAC system (with no re-circulating air to other apartments) to mitigate potential TB exposure to others.
Conclusion: It was necessary to engage a broad network of partners to achieve the patient’s optimal health and protect the community. TB programs can successfully leverage partnerships to access resources and support outside of public health.
Submitted by Wendy Noboa, BA, MS, PHA assigned to Virginia Department of Health and Arlington County, Department of Human Services, Public Health Division
Acknowledgements:
Our resilient patient ACPHD Staff: Wilson Coudon, MD; Melinda O’Brien, BSN, MA, RN; Monica Ferguson, MPH, RN; Sondra Dietz, MPH, MA; Evelyn Poppell, RN, MPA, Rachel Kidanne, MSN, RN; Crystal Sukhee, RN, BSN, Ana Caballero, RDN
Partners: Arlington Free Clinic; Virginia Department of Health, State TB Program; The Centers for Disease Control and Prevention; National Jewish Health; Virginia State Pharmacy; Arlington County’s Women, Infants and Children program, School Health program, Community Housing and Planning Department, and Economic Independence Bureau; and the University of Virginia Medical Center.
Laboratory Branch Welcomes New Reference Laboratory Team Lead

The DTBE Laboratory Branch is pleased to announce that Dr. Atanaska Marinova-Petkova has been selected as Dr. Beverly Metchock’s successor to serve as the Team Lead for the Reference Laboratory Team. Dr. Marinova-Petkova will provide supervision and leadership for LB’s CLIA-compliant testing including growth-based drug susceptibility testing and the Molecular Detection of Drug Resistance (MDDR) Service.
Dr. Marinova-Petkova has been an integral member of the LB Reference Laboratory Team since July 2019 with transition to a CLIA technical supervisor in September 2020. Prior to her time with DTBE, she obtained her DVM (2004), a MS in Molecular Biology and Biotechnology (2009), and a PhD in Epizootiology (2012). Atanaska completed a postdoctoral research appointment in the Department of Infectious Diseases at the St. Jude Children’s Research Hospital in Memphis before joining CDC as a Laboratory Advisor/Specialist in 2016. In 2017, she was selected as a CDC Laboratory Leadership Service (LLS) fellow for the Zoonoses and Select Agent Laboratory in the Bacterial Special Pathogens Branch. She has extensive technical laboratory experience including virology, bacteriology, serology, and molecular biology.
Submitted by Stephanie P. Johnston, MS
Congratulations to Dr. Beverly Metchock
With Dr. Beverly Metchock’s retirement at the end of September 2021, we would like to recognize her vast experience and knowledge of CLIA-compliant tuberculosis testing.

Dr. Metchock joined CDC in 1997 as a Supervisory Microbiologist and has served as Team Lead of the Reference Laboratory since 2004. She has been integral to advancing the state of TB laboratory testing in the United States and globally and has supported U.S. public health laboratories through her consultative leadership. Her clinical laboratory expertise, steadfast work ethic, and ability to systematically evaluate complex situations have led to continued advancements in our understanding of the genetic basis of drug resistance in Mycobacterium tuberculosis. Her efforts have aided standardizing phenotypic drug susceptibility testing (DST) methods. In 2009, Dr. Metchock and her team implemented a novel nationally available program for rapidly detecting mutations associated with drug resistant TB. This program, the CDC Molecular Detection of Drug Resistance (MDDR) Service, has been a model for global programs in establishing a national service that provides clear reporting language and expert consultation to aid results interpretation.
Dr. Metchock’s decades of work as a clinical microbiologist have enabled her to participate as part of multiple research collaborations, including support of the TB Trials Consortium, and as a subject-matter expert for numerous scientific panels. She has been a frequent presenter at international and national meetings. She has worked to educate public health experts, clinicians, and researchers about challenges associated with phenotypic DST, improving their understanding of the molecular basis of drug resistance in TB. Such understanding has improved rapid reporting of laboratory results for clinical care and more accurate surveillance for drug resistance.
While Dr. Metchock will be greatly missed at CDC and within the TB community, we want to thank her as well as congratulate her on her upcoming retirement. We wish her only the best in her future adventures.
*Picture of Beverly Metchock and Dan Ruggerio at NYC TB Program Review, 2008
Submitted by Stephanie P. Johnston, MS
Reported Tuberculosis in the United States, 2020 Now Available

On October 25, 2021 CDC published Reported Tuberculosis in the United States, 2020.
The 2020 data reveal a substantial decline in the number of reported cases of TB disease in the United States. The COVID-19 pandemic has probably affected reported TB incidence in the United States in several ways, including a combination of TB underdiagnosis and a true reduction in incidence. CDC is conducting additional analyses to better understand how the 2020 TB disease data compare with previous years.
Key Data Points:
- The reported number of TB cases in the United States declined from 8,904 TB cases in 2019 to 7,174 TB cases in 2020, a 19.4% decrease.
- The national TB incidence rate decreased from 2.7 cases per 100,000 persons in 2019 to 2.2 cases per 100,000 persons in 2020. Seven states and the District of Columbia reported TB disease incidence rates higher than the national average TB disease incidence rate.
- As in past years, cases of TB disease were not evenly distributed across the United States. Four states account for slightly more than half (50.3%) of all reported U.S. TB cases: California, Texas, New York (including New York City), and Florida.
In 2020, people from racial and ethnic minority groups and non-U.S. –born persons continued to be disproportionately affected by TB disease in the United States, highlighting the persistent health disparities and inequities among people with TB disease.
To support TB education and outreach to clinicians, health care agencies, and community organizations, CDC has created the following materials to highlight findings from the 2020 surveillance report:
Read more in a recent Dear Colleague Letter.
Submitted by Clarisse Tsang, MPH
Tuberculosis Epidemiologic Studies Consortium (TBESC)
In September 2021, DTBE launched the Tuberculosis Epidemiologic Studies Consortium III (TBESC-III), with the purpose of gaining a better understanding of how latent TB infection screening, testing, and treatment is offered to patients at risk for latent TB infection and TB disease in primary care settings, as well as the impact of interventions to increase the number of eligible patients who complete latent TB infection treatment. TBSEC-III aims to:
- Identify primary care systems/settings that serve non-U.S.–born persons at risk for latent TB infection and TB disease
- Collect retrospective and prospective electronic medical record data
- Design and implement clinical care-based interventions to improve performance measures across the latent TB infection care cascade
- Monitor and evaluate intervention performance over time to identify efficient and effective strategies.
TBESC is a CDC partnership with academic and clinical institutions and TB control programs to design, conduct, and evaluate programmatically relevant research (i.e., epidemiologic, behavioral, economic, laboratory, and operations) concerning the identification, diagnosis, prevention, and control of TB disease and latent TB infection. Organizations awarded contracts for TBESC-III are:
- Denver Health and Hospital Authority
- Kaiser Foundation Research Institute
- Public Health – Seattle & King County
- The Regents of the University of California, San Francisco
We look forward to TBESC’s contributions in identifying effective approaches to accelerate TB elimination in the United States.
Submitted by Justin Davis, MPH
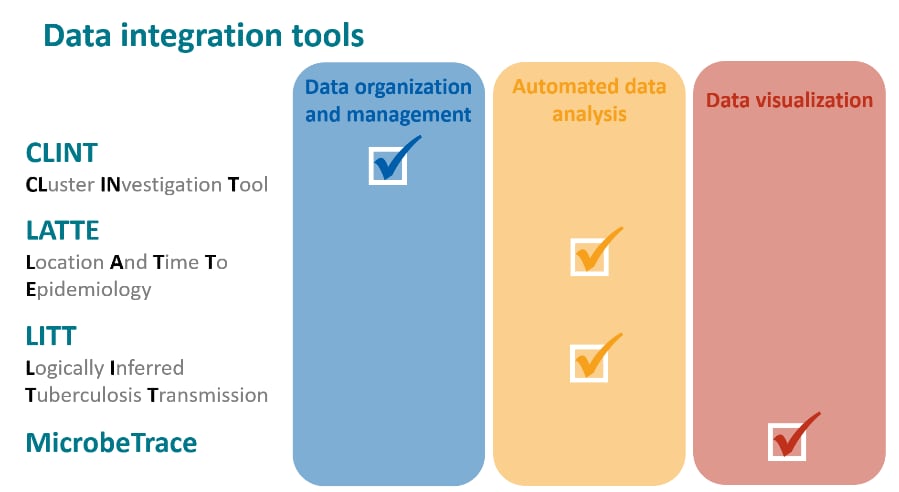
New Videos: TB Genotyping Analytic Tools
DTBE has produced a series of short videos to give an overview of data management and analysis tools that integrate whole-genome sequencing data with clinical and epidemiologic data for cluster investigation. The analytic tools, developed by DTBE, include the Cluster INvestigation Tool (CLINT), the Logically Inferred Tuberculosis Transmission (LITT) algorithm, and the Location And Time To Epidemiology (LATTE) algorithm. These tools aid in the organization, analysis, and visualization of data to help guide TB control efforts. Access these videos and tools by clicking ‘Analytic Tools’ on the newly redesigned TB genotyping website.
Submitted by Sarah Talarico and Clinton McDaniel
Notes from the Field: Tuberculosis Outbreak Linked to a Contaminated Bone Graft Product Used in Spinal Surgery — Delaware, March–June 2021
An article published in the September 10, 2021, issue of MMWR describes an investigation of a tuberculosis (TB) outbreak linked to a contaminated bone graft product. In May 2021, a Delaware hospital notified the Delaware Division of Public Health (DPH) of a TB cluster among 23 patients who had spinal surgery using a bone graft product.
An investigation by DPH, CDC and the U.S. Food and Drug Administration (FDA), found high rates of spinal and disseminated TB disease following implantation of the material. As of June 25, 19 patients (83%) had laboratory or imaging evidence of TB, 16 (70%) were hospitalized, 12 (52%) underwent additional procedures, and one died. Based on these findings, CDC recommended immediate treatment for TB disease for all patients nationwide who received the contaminated product and provided guidance to healthcare facilities to protect healthcare personnel and other patients who might have been exposed to M. tuberculosis during surgery and other patient care.
Learn more about the investigation in “Notes from the Field: Tuberculosis Outbreak Linked to a Contaminated Bone Graft Product Used in Spinal Surgery — Delaware, March–June 2021.”
Submitted by Noah Schwartz, MD
TB Elimination Alliance

The TB Elimination Alliance (TEA) has several resources and activities to help support their mission of eliminating TB among Asian American (AA) and Native Hawaiian/Pacific Islander (NH/PI) communities at risk. These activities include:
- Launched a new website (tbeliminationalliance.org), logo, and social media channels (@TBEAlliance on Twitter and Facebook).
- Hosted a webinar on “Addressing Latent Tuberculosis Infection and Tuberculosis in Asian and Native Hawaiian/Pacific Islander Communities: The Role of Health Centers for Testing and Treatment.”
- Announced 16 new mini-grant recipients to community providers and organizations to increase awareness and build capacity for latent TB infection and TB testing and treatment.
- Released TEA’s first Impact Report highlighting TEA’s progress towards eliminating TB.
To learn more about the TB Elimination Alliance, visit tbeliminationalliance.org.
Roadmap for Advancing TB Elimination in the United States through Scale up of Testing and Treatment of Latent TB Infection
Recommendations of the Advisory Council for the Elimination of Tuberculosis (ACET)
The Advisory Council for the Elimination of Tuberculosis (ACET) recently shared recommendations to prevent TB, specifically through focus on identifying individuals at risk and testing and treating latent TB infection. To scale up testing and treatment of latent TB infection, recommendations include: 1) identifying and engaging individuals at risk and their providers, 2) encouraging prevention using focused, effective testing and treatment strategies, 3) developing streamlined latent TB infection surveillance and monitoring systems to measure and optimize outcomes and 4) securing new funding to support these new activities.
The recommendations were published in ACET Minutes of the Meeting from June 16, 2020, Addendum 1, pages 40-51.
Tips for Coding and Billing: Screening, Diagnosis, and Treatment of Latent Tuberculosis Infection in Primary Care Settings.
Heartland National TB Center has released a new product covering tips for coding and billing associated with management of patients with latent TB infection. The product, entitled Screening, Diagnosis, and Treatment of Latent Tuberculosis Infection in Primary Care Settings is intended for use by physicians and nurses in primary care settings but can be used in any instance where accurate coding is desired.
This product provides basic information regarding screening, diagnosing, testing, evaluating, treatment regimens, monitoring, initial visits, directly observed therapy and monthly visits. In addition, it provides direct links to more comprehensive guidance on these topics.
Heartland provides a visual diagram to guide the user through the entire screening process. CPT and ICD codes are provided for commonly seen TB symptoms and tests. It also provides steps for unexpected situations and points of contact.
For more information on the Tips for Billing and Coding product, please visit the products page on our Heartland website https://www.heartlandntbc.org/products/
Cha S, Henry A, Montgomery MP, Laws RL, Pham H, Wortham J, Garg S, Kim L, Mosites E; COVID-NET Surveillance Team. Morbidity and mortality among adults experiencing homelessness hospitalized with COVID-19. J Infect Dis 2021:jiab261. Epub ahead of print.
Chu VT, Yousaf AR, Chang K, Schwartz NG, McDaniel CJ, Lee SH, Szablewski CM, Brown M, Drenzek CL, Dirlikov E, Rose DA, Villanueva J, Fry AM, Hall AJ, Kirking HL, Tate JE, Lanzieri TM, Stewart RJ; Georgia Camp Investigation Team. Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med 2021. Epub ahead of print.
Dawood FS, Varner M, Tita A, Newes-Adeyi G, Gyamfi-Bannerman C, Battarbee A, Bruno A, Daugherty M, Reichle L, Vorwaller K, Vargas C, Parks M, Powers E, Lucca-Susana M, Gibson M, Subramaniam A, Cheng YJ, Feng PJ, Ellington S, Galang RR, Meece J, Flygare C, Stockwell MS. Incidence, clinical characteristics, and risk factors of SARS-CoV-2 infection among pregnant individuals in the United States. Clin Infect Dis 2021:ciab713. Epub ahead of print.
Fields VL, Kiphibane T, Eason JT, Hafoka SF, Lopez AS, Schwartz A, Henry A, Tran CH, Tate JE, Kirking HL, Laws RL, Venkatappa T, Mosites E, Montgomery MP. Assessment of contact tracing for COVID-19 among people experiencing homelessness, Salt Lake County Health Department, March–May 2020. Ann Epidemiol 202l;59:50–55.
Garg S, Patel K, Pham H, Whitaker M, O’Halloran A, Milucky J, Anglin O, Kirley PD, Reingold A, Kawasaki B, Herlihy R, Yousey-Hindes K, Maslar A, Anderson EJ, Openo KP, Weigel A, Teno K, Ryan PA, Monroe ML, Reeg L, Kim S, Como-Sabetti K, Bye E, Shrum Davis S, Eisenberg N, Muse A, Barney G, Bennett NM, Felsen CB, Billing L, Shiltz J, Sutton M, Abdullah N, Talbot HK, Schaffner W, Hill M, Chatelain R, Wortham J, Taylor C, Hall A, Fry AM, Kim L, Havers FP. Clinical trends among U.S. adults hospitalized with COVID-19, March to December 2020 : A cross-sectional study. Ann Intern Med 2021. Epub ahead of print.
Godfred-Cato S, Tsang CA, Giovanni J, Abrams J, Oster ME, Lee EH, Lash MK, Le Marchand C, Liu CY, Newhouse CN, Richardson G, Murray MT, Lim S, Haupt TE, Hartley A, Sosa LE, Ngamsnga K, Garcia A, Datta D, Belay ED. Multisystem inflammatory syndrome in infants <12 months of age, United States, May 2020–January 2021. Pediatr Infect Dis J 2021;40:601–05.
Goswami ND, Fiore AE, Walke HT. Evidence, experience, expertise, and the US coronavirus disease 2019 public health response. Clin Infect Dis 2021;73:S1–S4.
Havers FP, Whitaker M, Self JL, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Weigel A, Teno K, Monroe ML, Ryan PA, Reeg L, Kohrman A, Lynfield R, Como-Sabetti K, Poblete M, McMullen C, Muse A, Spina N, Bennett NM, Gaitán M, Billing LM, Shiltz J, Sutton M, Abdullah N, Schaffner W, H. Keipp Talbot H, Crossland M, George A, Patel K, Pham H, Milucky J, Anglin O, Ujamaa D, Hall AJ, Garg S, Taylor CA, COVID-NET Surveillance Team. Hospitalization of adolescents aged 12–17 years with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 1, 2020–April 24, 2021. MMWR Morb Mortal Wkly Rep 2021;70:851–57.
Lawandi A, Warner S, Sun J, Demirkale CY, Danner RL, Klompas M, Gundlapalli A, Datta D, Harris AM, Morris SB, Natarajan P, Kadri SS. Suspected SARS-CoV-2 reinfections: Incidence, predictors, and healthcare use among patients at 238 U.S. healthcare facilities, June 1, 2020–February 28, 2021. Clin Infect Dis 2021:ciab671. Epub ahead of print.
Lee JT, Althomsons SP, Wu H, Budnitz DS, Kalayil EJ, Lindley MC, Pingali C, Bridges CB, Geller AI, Parker Fiebelkorn A, Graitcer SB, Singleton JA, Patel SA. Disparities in COVID-19 vaccination coverage among health care personnel working in long-term care facilities, by job category, National Healthcare Safety Network — United States, March 2021. MMWR Morb Mortal Wkly Rep 2021;70:1036–39.
Marks SM, Clara A, Fiebelkorn AP, Le X, Armstrong PA, Campbell S, Van Alstyne JM, Price S, Bolton J, Sandhu PK, Bombard JM, Strona FV. Influenza vaccination in health centers during the coronavirus disease 2019 pandemic—United States, 7–27 November 2020. Clin Infect Dis 2021;73:S92–S97.
Self JL, Montgomery MP, Mosites E. Self et al. respond. Am J Public Health 2021;111(8):e14.
Rogers-Brown JS, Wanga V, Okoro C, Brozowsky D, Evans A, Hopwood D, Cope JR, Jackson BR, Bushman D, Hernandez-Romieu AC, Bonacci RA, McLeod T, Chevinsky JR, Goodman AB, Dixson MG, Lufty C, Rushmore J, Koumans E, Bamrah Morris S, Thompson W. Outcomes among patients referred to outpatient rehabilitation clinics after COVID-19 diagnosis — United States, January 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021;70:967–71.
Shragai T, Smith-Jeffcoat SE, Koh M, Schechter MC, Rebolledo PA, Kasinathan V, Wang Y, Hoffman A, Miller H, Tejada-Strop A, Jain S, Tamin A, Harcourt JL, Thornburg NJ, Wong P, Medrzycki M, Folster JM, Semenova V, Steward-Clark E, Drobenuic J, Biedron C, Stewart RJ, da Silva J, Kirking HL, Tate JE; CDC COVID-19 Emergency Response GA-10 Field Team. Epidemiologic, immunologic, and virus characteristics in patients with paired SARS-CoV-2 serology and reverse transcription polymerase chain reaction testing. J Infect Dis 2021:jiab349. Epub ahead of print.
Siegel DA, Reses HE, Cool AJ, Shapiro CN, Hsu J, Boehmer TK, Cornwall CR, Gray EB, Henley SJ, Lochner K, Suthar AB, Lyons C, Mattocks L, Hartnett K, Adjemian J, van Santen KL, Sheppard M, Soetebier KA, Logan P, Martin M, Idubor O, Natarajan P, Sircar K, Oyegun E, Dalton J, Perrine CG, Peacock G, Schweitzer B, Bamrah Morris S, Raizes E. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents Aged 0–17 Years—United States, August 2020–August 2021. MMWR Morb Mortal Wkly Rep 2021;70(36):1249–54.
Wadhwa A, Yin S, Freeman B, Hershow RB, Killerby M, Yousaf AR, Lester S, Mills L, Buono SA, Pomeroy M, Owusu D, Chu VT, Tate JE, Bhattacharyya S, Hall P, Thornburg NJ, Kirking HL. Comparison of the SARS-CoV-2 spike protein ELISA and the Abbott Architect SARS-CoV-2 IgG nucleocapsid protein assays for detection of antibodies. PLoS One. 2021 Jul 29;16(7):e0255208.
Cegielski JP, Udwadia ZF, Viiklepp P, Yim JJ, Menzies D. Reply to van Deun and Decroo. Clin Infect Dis 2021;72:e1168-e1169.
Gewitz AD, Solans BP, Mac Kenzie WR, Heilig C, Whitworth WC, Johnson JL, Nsubuga P, Dorman S, Weiner M, Savic RM; Tuberculosis Trials Consortium of the Centers for Disease Control Prevention. A longitudinal model-based biomarker analysis of exposure response in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2021:AAC0179420. Epub ahead of print.
Ho CS, Feng PI, Narita M, Stout JE, Chen M, Pascopella L, Garfein R, Reves R, Katz DJ; Tuberculosis Epidemiologic Studies Consortium. Comparison of three tests for latent tuberculosis infection in high-risk people in the USA: an observational cohort study. Lancet Infect Dis 2021. Epub ahead of print.
Kim S, Cohen T, Horsburgh CR, Miller JW, Hill AN, Marks SM, Li R, Kammerer JS, Salomon JA, Menzies NA. Trends, mechanisms, and racial/ethnic differences of tuberculosis incidence in the US-born population aged 50 years or older in the United States. Clin Infect Dis 2021. Epub ahead of print.
Largen A, Ayala A, Khurana R, Katz DJ, Venkatappa TK, Brostrom R. Evaluation of point-of-care algorithms to detect diabetes during screening for latent TB infection. Int J Tuberc Lung Dis 2021;25:547–53.
Li R, Wilson WW, Schwartz NG, Hernandez-Romieu AC, Glowicz J, Hanlin E, Taylor M, Pelkey H, Briody CA, Gireesh L, Eskander M, Lingenfelter K, Althomsons SP, Stewart RJ, Free R, Annambhotla P, Basavaraju SV, Wortham JM, Bamrah Morris S, Benowitz I, Haddad MB, Hong R, Drees M. Notes from the field: tuberculosis outbreak linked to a contaminated bone graft product used in spinal surgery—Delaware, March–June 2021. MMWR Morb Mortal Wkly Rep 2021;70(36):1261–3.
Narita M, Hatt G, Gardner Toren K, Vuong K, Pecha M, Jereb JA, Goswami ND. Delayed tuberculosis diagnoses during the coronavirus disease 2019 (COVID-19) pandemic in 2020—King County, Washington. Clin Infect Dis 2021;73:S74–S76.
Parmer J, Macario E, Tatum K, Brackett A, Allen L, Picard R, DeLuca N, Dowling M. Latent tuberculosis infection: misperceptions among non-U.S.-born-populations from countries where tuberculosis is common. Glob Public Health 2021:1–15. Epub ahead of print.
Springer YP, Kammerer JS, Silk BJ, Langer AJ. Tuberculosis in indigenous persons—United States, 2009–2019. J Racial Ethn Health Disparities 2021. Epub ahead of print.
Winglee K, McDaniel CJ, Linde L, Kammerer S, Cilnis M, Raz KM, Noboa W, Knorr J, Cowan L, Reynolds S, Posey J, Sullivan Meissner J, Poonja S, Shaw T, Talarico S, Silk BJ. Logically inferred tuberculosis transmission (LITT): a data integration algorithm to rank potential source cases. Front Public Health 2021;9:667337.
Wortham JM, Li R, Althomsons SP, Kammerer S, Haddad MB, Powell KM. Tuberculosis genotype clusters and transmission in the U.S., 2009–2018. Am J Prev Med 2021:S0749-3797(21)00165-3. Epub ahead of print.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Meredith Moore at mue1@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.