TB NOTES

TB Notes 3, 2023
Dear Colleague,
It was an exciting and engaging summer for the Division of Tuberculosis Elimination (DTBE). We are happy to welcome Dr. Mandy Cohen as the 20th Director of CDC and look forward to working with her toward TB elimination in the United States.

Last week, the United Nations General Assembly (UNGA) held the second high-level meeting on TB. The theme of the meeting was: “Advancing science, finance and innovation, and their benefits, to urgently end the global tuberculosis epidemic, in particular, by ensuring equitable access to prevention, testing, treatment and care.” CDC, along with global and domestic TB partners, also came together at a side event prior to the meeting.
Earlier this month, CDC released new TB resources to increase awareness about the TB screening and attestation requirements of the Uniting for Ukraine program. The materials can be adapted to promote local TB testing events, resources, and share information for arrivals and their sponsors.

Additionally, CDC is proud to continue our support of the TB Elimination Alliance (TEA), which recently awarded a third cycle of 12 mini-grant awards totaling $250,000 to community providers that increase awareness and build capacity on TB testing and treatment. These community health organizations serve Asian, Asian American, Native Hawaiian, and Pacific Islander, Hispanic/Latino and African American communities that are disproportionately impacted by TB.
These are just a few highlights from our work over the past quarter. I invite you to read further for additional news and resources from across DTBE.
Thank you for your continued efforts towards our goal of TB elimination.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV, Viral Hepatitis, STD, and TB Prevention
Fact Sheet: What You Need to Know About the TB Skin Test

What You Need to Know About the TB Skin Test fact sheet is now available in English and Spanish. This fact sheet can be used to provide information to persons that are receiving a TB skin test. The fact sheet describes the TB skin test, includes space to write return appointment details, and explains TB skin test results.
The fact sheet can be downloaded from the CDC TB website. Print copies are available to order free of charge on the CDC On Demand website. For more information on the fact sheet and CDC’s additional Mantoux tuberculin skin testing products, please visit the CDC website.
Submitted by Coralis Rodriguez Morales, MPH, Carissa Sera-Josef, MPH, and Peri Hopkins, MPH
Uniting for Ukraine Partner Toolkit

CDC is pleased to announce the release of new TB resources to increase awareness about the TB screening and attestation requirements of the Uniting for Ukraine program. Resources are available in Ukrainian, Russian, and English and include a conversation guide, fact sheet, flyer, and poster. Partners can also adapt sample social media content, text message, and newsletter content to share information with people arriving from Ukraine and their sponsors. Print copies are available to order free of charge on the CDC On Demand website.
We encourage you to use the Uniting for Ukraine resources in your communication and outreach efforts about the Uniting for Ukraine TB screening and attestation requirement.
Submitted by Meredith Moore, MPH
Tuberculosis Infection among Non–U.S.-Born Persons and Persons ≥60 Years of Age, United States, 2019–2020

Beginning in 1970, CDC funded a TB component of several National Health and Nutrition Examination Surveys (NHANES) that provide the primary source of national data on latent TB infection in the U.S. population. Most recently, CDC provided funding for the 2019–2020 NHANES survey to examine latent TB infection among non-U.S.–born persons >6 years of age and all persons >60 years of age. Due to the COVID-19 pandemic, only a partial 2019-2020 NHANES dataset was available for analysis and the results were not representative of the entire U.S. population.
From 2019 to 2020, 10% of non-U.S.–born and 7% of study participants 60 years of age and above tested positive for TB infection. Although not nationally representative, study data still indicate that TB infection remains a critical health screening consideration among non-U.S.–born persons and persons less than 60 years of age. Visit the CDC website to learn more about the 2019-2020 NHANES TB infection results.
Submitted by Rachel Woodruff, MPH
Field Services Branch (FSB) Welcomes Dr. Caitlin Reed, MD, MPH

Caitlin Reed, MD, MPH has joined FSB as the new Medical Team Lead. Dr. Reed is an infectious disease specialist and TB subject matter expert with extensive TB clinical experience and public health training. She completed medical school at Harvard, internship at UCSF, and residency in internal medicine at Olive View-UCLA Medical Center Dr. Reed earned an MPH in epidemiology at Johns Hopkins as a Sommer Scholar and completed a thesis on TB diagnostics after completing a fellowship in infectious diseases at Harbor-UCLA Medical Center. She served for two years as a CDC EIS Officer assigned to Los Angeles County after earning an MPH.
Dr. Reed became the founding Medical Director of the Inpatient TB Unit at Olive View-UCLA Medical Center in 2011. There, she led a multidisciplinary team caring for patients with severe and disseminated TB disease, TB meningitis, HIV/TB coinfection, drug-resistant TB, substance use and mental health issues, and nonadherence with TB treatment requiring legal orders. At the Los Angeles County Department of Health Services, she developed a TB educational curriculum for Infectious Disease fellows and medicine residents and implemented new expected practices to integrate short course latent TB infection treatment into primary care practices across a large urban safety-net medical system.
Submitted by Terry Chorba, MD, DSc, LLM, FACP, FIDSA
Washington, D.C. “Join the fight to stop TB” Campaign

The DC Health and Wellness Center offers free TB testing and evaluation for Uniting for Ukraine beneficiaries and others. To increase awareness of these services, April Cabos, Public Health Advisor, and the team in Washington, D.C. have launched the “Join the fight to stop TB” campaign to increase awareness of TB.
The campaign materials can be found in several metro stations and bus shelters, as well as on social media. The campaign is also available in multiple languages. To learn more about TB risk factors and symptoms as well as resources in D.C., visit the DC Health web page.
Submitted by April Cabos
Rapid Laboratory Testing for Fluoroquinolone (FQ) Resistance
Beginning May 24, 2023, in response to reported drug shortages and to support programmatic implementation of the alternate 4-month rifapentine-moxifloxacin (RPT-MOX) regimen, DTBE Lab Branch implemented rapid testing for fluoroquinolone (FQ) resistance. Please note the following:
- Acceptable samples include nucleic-acid amplification test-positive sediments and Mycobacterium tuberculosis isolates with a strong preference for submission of isolates given that sediments with low bacterial burden are less likely to be successfully tested.
- Samples received for rapid FQ testing will only be assessed by CLIA-compliant molecular methods. The sequencing panel used will also include examination of genetic loci associated with resistance to rifampin, isoniazid, and pyrazinamide.
- Given unknowns regarding the potential volume of requests for rapid FQ testing and the need to assure sufficient resources to meet testing needs, phenotypic drug susceptibility testing (DST) will not generally be performed.
- Samples with detected reportable mutations in genetic loci associated with rifampin and FQ resistance will be reflexed for the full Molecular Detection of Drug Resistance (MDDR) testing panel, along with concurrent growth-based DST.
- To ensure compliance with the laboratory’s CLIA acceptance criteria, submissions will be subject to the same preapproval process as MDDR.
- To request rapid FQ testing, please indicate “rapid FQ testing needed” in Section 3: Submission Criteria under “Other” in the MDDR Request Form and indicate if the request is due to drug shortages or use of the alternate 4-month RPT-MOX regimen. Providing this information is critical to ensure appropriate laboratory workflow.
- Submitting laboratories should use test order number 10191 (Mycobacterium TB Complex – Special Study) on the CDC Test Requisition Form (50.34).
Routine MDDR testing using the full panel of genetic loci remains available for cases where drug resistance is suspected or known. If you have any questions regarding CDC’s services, please feel free to reach out to TBLab@cdc.gov. Please refer to the MDDR User Guide for additional submission requirements. Depending on capacity and resources, laboratories are encouraged to explore implementation of in-house testing for FQs.
Public health laboratories submitting samples to the DST Reference Center at the California Microbial Diseases Laboratory (MDL) should continue to use that mechanism. For questions related to testing at the DST Reference Center at MDL, please reach out to cdphtbdst@cdph.ca.gov.
Submitted by Angela Starks, PhD
New Continuity of Operations Plan Toolkit

CDC’s Division of Tuberculosis Elimination Laboratory Branch is excited to announce the newly available “Continuity of Operations Plan Toolkit for Public Health Mycobacteriology Laboratories.”
This toolkit focuses on continuity of operations plans (COOP) for mycobacteriology laboratory testing and the continuity of testing services for laboratory diagnosis of TB. Interruption of service events may result in a temporary inability to use equipment or laboratory space, compromise staffing and infrastructure, preclude maintenance or calibration of equipment, and prevent or require extensive disinfection and decontamination. A COOP allows a mycobacteriology laboratory to shift efficiently from a regular structure to one that supports the timely continuation of services.
The toolkit includes processes for creating, modifying, or implementing mycobacteriology COOPs, including considerations for leveraging partnerships. Templates, checklists, and contact lists are also included that are editable and adaptable for local needs. The toolkit can be found on the CDC website.
While intended for public health laboratories, this toolkit has applicability for clinical, commercial, or other laboratories that perform mycobacteriology testing. Please share this new resource with your colleagues and partners.
Submitted by Monica Youngblood, MPH, M(ASCP) and Stephanie Johnston
Latent TB Infection Law Map
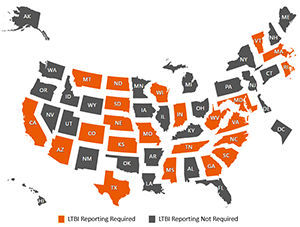
The DTBE Surveillance Team partnered with the CDC Office of Public Health Law Services to complete a legal epidemiology assessment of statutes and regulations associated with latent TB infection reporting across 50 states and the District of Columbia. In each of these jurisdictions, all cases of TB disease are required to be reported to local or state health authorities and the CDC. Reporting latent TB infection to CDC is optional, but many states require reporting.
This analysis concluded that 23 of 51 (45%) jurisdictions have implemented jurisdiction-level legal reporting requirements as a tool to prevent TB disease. Of these, 14 (61%) require that all latent TB infection cases be reported, 6 (26%) specify a laboratory test requirement (such as positive TB blood test) or restrict reporting to certain conditions (such as correctional facility workers), and 3 (13%) restrict reporting to specific age requirements. The project summary, map, and detailed table by jurisdiction is available on the Latent TB Infection Resource Hub.
Submitted by Erin Lee Miller, PhD
Tuberculosis Elimination Alliance Awards Mini-Grants for Tuberculosis Testing, Treatment, and Prevention

The Tuberculosis Elimination Alliance (TEA), led by the Association of Asian Pacific Community Health Organizations (AAPCHO), Asian & Pacific Islander American Health Forum (APIAHF), Hepatitis B Foundation (HBF), and Stop TB USA, with the support of CDC, announced a third cycle of 12 mini-grant awards totaling $250,000 to community providers that increase awareness and build capacity on TB testing and treatment. The newly awarded mini-grant recipients in this cycle are as follows:
- Arkansas Coalition of Marshallese (Springdale, AR)
- Asian Pacific Health Foundation (San Diego, CA)
- EthnoMed (Seattle, WA)
- Jericho Road Community Health Center (Buffalo, NY)
- Midwest Asian Health Association (Chicago, IL)
- Mission Neighborhood Health Center (San Francisco, CA)
- Regional Pacific Islander Taskforce (San Francisco, CA)
- Revive Community Health (Tempe, AZ)
- Rural Women’s Health Project (Gainesville, FL)
- San Diego County Medical Society Foundation, dba Champions for Health (San Diego, CA)
- Todu Guam Foundation, Ltd. (Tamuning, Guam)
- We Are TB / Somos TB (National)
Since its inception in 2019, TEA has distributed over $800,000 in mini-grant funding to 36 organizations. TEA, its members, partners, and mini-grantees look forward to building on the progress towards TB elimination.
For more information, visit the TEA website.
2023 TEA Virtual Summit: Building Healthy Communities Free of TB
TEA invites you to join the 2023 virtual annual summit on November 8-9, 2023. This exciting event will take place via Zoom from 9 am to 2 pm PT / 12 pm to 5 pm ET. This year’s summit will center around the theme of showcasing TEA’s Mini-Grant Program on “Building Healthy Communities Free of TB,” and the remarkable innovations that have emerged from diverse communities and their collaborative efforts towards achieving health equity over the past five years. Registration for the annual summit is now available.
Civil Surgeon and Panel Physician Workshop

In August, CDC participated in the Civil Surgeon and Panel Physicians Workshop in Atlanta, Georgia. The theme of the workshop was “Bridging the Gap: Supporting Healthy Migration”. DTBE shared information during several presentations and hosted an exhibit booth with resources from the Think. Test. Treat TB campaign.
Cash McGinley HL, Hancock WT, Kern-Allely S, Jenssen M, Chutaro E, Camacho J, Judicpa P, Okumura K, Muñoz N, Ademokun OM, Brostrom R. COVID-19 in the US-affiliated Pacific Islands: A timeline of events and lessons learned from March 2020-November 2022. PLOS Glob Public Health. 2023 Aug 16;3(8):e0002052.
Groenweghe E, Swensson L, Winans KD, Griffin P, Haddad MB, Brostrom RJ, Tuckey D, Lam CK, Armitige LY, Seaworth BJ, Corriveau EA. Outbreak of Multidrug-Resistant Tuberculosis — Kansas, 2021–2022. MMWR Morb Mortal Wkly Rep 2023; 72:957-960.
Swartwood NA, Testa C, Cohen T, Marks SM, Hill AN, Beeler Asay G, Cochran J, Cranston K, Randall LM, Tibbs A, Horsburgh CR Jr, Salomon JA, Menzies NA. Tabby2: a user-friendly web tool for forecasting state-level TB outcomes in the United States. BMC Med. 2023 Aug 30;21(1):331.
Adams T, Miller K, Law M, Pitcher E, Chinpar B, White K, Deutsch-Feldman M, Li R, Filardo TD, Romieu-Hernandez AC, Schwartz NG, Haddad MB, Glowicz J. Systematic contact investigation: an essential infection prevention skill to prevent tuberculosis transmission in healthcare settings. Am J Infect Control. 2023 Jun 22:S0196-6553(23)00478-9.
Feng PI, Horne DJ, Wortham JM, Katz DJ; CDC Tuberculosis Epidemiologic Studies Consortium. Trends in tuberculosis clinicians’ adoption of short-course regimens for latent tuberculosis infection. J Clin Tuberc Other Mycobact Dis. 2023 Jun 13;33:100382.
Ortega-Villa AM, Hynes NA, Levine CB, Yang K, Wiley Z, Jilg N, Wang J, Whitaker JA, Colombo CJ, Nayak SU, Kim HJ, Iovine NM, Ince D, Cohen SH, Langer AJ, Wortham JM, Atmar RL, El Sahly HM, Jain MK, Mehta AK, Wolfe CR, Gomez CA, Beresnev T, Mularski RA, Paules CI, Kalil AC, Branche AR, Luetkemeyer A, Zingman BS, Voell J, Whitaker M, Harkins MS, Davey RT Jr, Grossberg R, George SL, Tapson V, Short WR, Ghazaryan V, Benson CA, Dodd LE, Sweeney DA, Tomashek KM. Evaluating Demographic Representation in Clinical Trials: Use of the Adaptive Coronavirus Disease 2019 Treatment Trial (ACTT) as a Test Case. Open Forum Infect Dis. 2023 May 27;10(6):ofad290.
Venkatappa T, Shen D, Ayala A, Li R, Sorri Y, Punnoose R, Katz D; Tuberculosis Epidemiologic Studies Consortium. Association of Mycobacterium tuberculosis infection test results with risk factors for tuberculosis transmission. J Clin Tuberc Other Mycobact Dis. 2023 Jun 29;33:100386.
Baker JJ, Nahar R, Petroelje BK, Goswami ND, Lardizabal AA. Fluoroquinolone-resistant latent tuberculosis infection: A literature review and case series of 5 patients treated with linezolid monotherapy. J Clin Tuberc Other Mycobact Dis 2023;32:100376.
Haley CA, Schechter MC, Ashkin D, Peloquin CA, Cegielski JP, Andrino BB, Burgos M, Caloia LA, Chen L, Colon-Semidey A, DeSilva MB, Dhanireddy S, Dorman SE, Dworkin FF, Hammond-Epstein H, Easton AV, Gaensbauer JT, Ghassemieh B, Gomez ME, Horne D, Jasuja S, Jones BA, Kaplan LJ, Khan AE, Kracen E, Labuda S, Landers KM, Lardizabal AA, Lasley MT, Letzer DM, Lopes VK, Lubelchek RJ, Macias CP, Mihalyov A, Misch EA, Murray JA, Narita M, Nilsen DM, Ninneman MJ, Ogawa L, Oladele A, Overman M, Ray SM, Ritger KA, Rowlinson MC, Sabuwala N, Schiller TM, Schwartz LE, Spitters C, Thomson DB, Tresgallo RR, Valois P, Goswami ND; BPaL Implementation Group. Implementation of BPaL in the United States: experience using a novel all-oral treatment regimen for treatment of rifampin-resistant or rifampin-intolerant TB disease. Clin Infect Dis 2023:ciad312. Epub ahead of print.
Scott NA, Sadowski C, Vernon A, Arevalo B, Beer K, Borisov A, Cayla JA, Chen M, Feng PJ, Moro RN, Holland DP, Martinson N, Millet JP, Miro JM, Belknap R. Using a medication event monitoring system to evaluate self-report and pill count for determining treatment completion with self-administered, once-weekly isoniazid and rifapentine. Contemp Clin Trials 2023;129:107173.
Zavala S, Winglee K, Ho CS, Pettit AC, Ahmed A, Katz DJ, Belknap RW, Stout JE; Tuberculosis Epidemiologic Studies Consortium. Examining test cutoffs to optimize diagnosis of latent tuberculosis infection in non-US-born people. Ann Am Thorac Soc 2023. Epub ahead of print.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Kevin Crooks at qyd7@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.