TB NOTES

TB Notes 1, 2022
Notes from the Director

Dear Colleague,
It has been an exciting first quarter for the Division of Tuberculosis Elimination (DTBE), with the release of several new publications, guidelines, a new TB communications campaign, and other projects.
In January, CDC and collaborators published “In-Person vs Electronic Directly Observed Therapy for Tuberculosis Treatment Adherence” in the Journal of the American Medical Association (JAMA) Network Open, showing that electronic directly observed therapy (eDOT) was as effective as traditional in-person DOT for ensuring high adherence to tuberculosis (TB) treatment. In February, CDC released “Provisional CDC Guidance for the Use of Pretomanid as part of a Regimen [Bedaquiline, Pretomanid, and Linezolid (BPaL)] to Treat Drug-Resistant Tuberculosis Disease” and interim guidance for a 4-month treatment regimen to treat drug-susceptible TB disease in the United States. The 4-month regimen containing rifapentine, moxifloxacin, isoniazid, and pyrazinamide is the first successful short treatment regimen for drug-susceptible TB disease identified in almost 40 years.
Last week, we recognized World TB Day on March 24th to commemorate the date Dr. Robert Koch announced his discovery of the bacillus that causes TB. This is the third World TB Day we have observed amid the COVID-19 pandemic, and many of our colleagues across the country and around the world continue to support the pandemic response. I am inspired by the stories of CDC’s 2022 U.S. TB Elimination Champions, and want to thank them, as well as the public health and medical professionals on the frontlines responding to the COVID-19 pandemic while continuing to work towards TB elimination.
On March 24, 2022, CDC published provisional 2021 U.S. TB surveillance data. Reported TB incidence rose 9.4% during 2021 (2.4 cases per 100,000 persons) compared with 2020 (2.2 cases per 100,000 persons) but was lower than TB incidence in 2019 (2.7 cases per 100,000 persons). Concerns remain about delayed or missed TB diagnoses associated with the COVID-19 pandemic. Timely diagnosis and treatment for TB and latent TB infection remain critical to achieving elimination.
CDC is partnering with clinicians, healthcare agencies, and community organizations, especially those serving populations at increased risk for TB, through a new campaign: Think. Test. Treat TB. This is the first national federally-funded communications campaign to increase testing and treatment for latent TB infection in the United States. The campaign website includes resources for providers, patients, and community organizations.
These are just a few highlights from our work over the past quarter. I invite you to read further for additional news, resources, and success stories from across DTBE.
Thank you for your continued efforts towards our goal of TB elimination.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Welcome Wendy Carr, Ph.D., Chief of Clinical Research Branch

Wendy Carr, Ph.D., has been selected as the Chief of the Clinical Research Branch (CRB) and officially assumed this role on March 1, 2022.
Wendy has served as CRB’s Design and Oversight Team Lead since 2018. Under her leadership, the competitive process for new TB Trials Consortium (TBTC) contracts was successfully accomplished, resulting in the formation of a strong Consortium for the next ten-year research cycle. She recently led the development of CDC interim guidance for the 4-month rifapentine-moxifloxacin regimen for treatment of drug-susceptible pulmonary TB. She has more than 15 years of experience in clinical trials and human subjects protection, including serving as the Regulatory Oversight Team Lead and subsequently the Principal Investigator for the STRIVE trial (Sierra Leone Trial to Introduce a Vaccine against Ebola), where she was responsible for diverse aspects of trial implementation, regulatory guidance and interpretation for clinical trial operations. At CDC, she has also served in the Advisory Committee on Immunization Practices (ACIP) secretariat focused on evidence-based methodology for developing public health recommendations, as a Human Subjects Advisor guiding investigators on regulations pertaining to human subjects protection, and as a Scientific Review Officer providing oversight of peer review of research grants and cooperative agreements.
Submitted by Carla Jeffries, JD, MPH
CDC Releases Interim Guidance on a Four-month TB Treatment Regimen

Shorter treatment regimens for TB disease can be more convenient and help patients finish treatment faster. On February 24, 2022, CDC released new interim guidance in Morbidity and Mortality Weekly Report for a 4-month treatment regimen to treat drug-susceptible TB disease in the United States. The 4-month rifapentine-moxifloxacin regimen, which is as effective as the standard 6-month regimen for TB treatment, is a treatment option for people 12 years of age and older with drug-susceptible pulmonary TB disease in the United States. The 4-month treatment regimen consists of high-dose daily rifapentine with moxifloxacin, isoniazid, and pyrazinamide for 8 weeks, followed by 9-weeks of daily rifapentine, moxifloxacin, and isoniazid.
The new guidance is based on resultsexternal icon from an international phase 3 clinical trial, sponsored by CDC’s Tuberculosis Trials Consortium and conducted in collaboration with the National Institutes of Health-sponsored AIDS Clinical Trials Group, which confirmed that a 4-month daily treatment regimen with high-dose rifapentine and moxifloxacin is as effective as (noninferior to) the standard daily 6-month regimen in curing drug-susceptible TB. This important study was recognized as one of the New England Journal of Medicine’s (NEJM’s) 2021 Notable Articlesexternal icon. The “Notable Articles” are selected by NEJM editors as the most meaningful in changing medical practice and improving patient care.
CDC has resources on the 4-month rifapentine-moxifloxacin regimen for healthcare providers and TB programs, including
- An updated Treatment of TB Disease webpage that includes a regimen table, considerations for specific groups of people with TB disease, and a comparison table of the recommended evaluation and testing considerations for the TB disease treatment regimens,
- A checklistpdf icon to ensure the correct timing of testing and monitoring for patients through the regimen, and
- A Frequently Asked Questions (FAQ) webpage for providers to address common questions about the 4-month regimen.
Healthcare providers can contact their State TB Control Offices and the TB Centers of Excellence for Training, Education, and Medical Consultation for additional information about this TB treatment regimen and support in treating people with TB disease.
Submitted by Wendy Carr, PhD
Using pharmacogenetics to enhance tuberculosis (TB) treatment
Through a funding opportunity from CDC’s Office of Genomics and Precision Public Health in collaboration with the CDC Office of Advanced Molecular Detection, DTBE will conduct a 2-year project to assess relationships between pharmacogenetics (PG), TB drug exposure, relevant treatment outcomes, and safety.
Researchers will use information collected in an international phase 3 clinical trialexternal icon led by CDC’s Tuberculosis Trials Consortium (TBTC), in collaboration with the National Institutes of Health-sponsored AIDS Clinical Trial Group. A total of 2,516 participants at 34 clinical sites in 13 countries participated in the trial, which demonstrated that a 4-month daily treatment regimen with high-dose rifapentine and moxifloxacin is as effective as (noninferior to) the standard daily 6-month regimen in curing drug-susceptible TB disease.
Samples for PG analyses collected from a large sub-set of 1,818 trial participants will be genotyped followed by genetic association analyses. This project aims to evaluate the contribution of pharmacogenetics to patient efficacy and safety outcomes by incorporating PG data into population pharmacokinetics (PK) models for six major TB drugs and risk algorithms for unfavorable treatment outcomes and to develop and evaluate PG-based dosing algorithms for novel high-dose rifapentine-based regimens. Accounting for PG can reduce unexplained variability in PK, eliminating the need to measure individual drug levels to predict outcomes, improving the ability of current algorithms to identify patients at high risk of unfavorable outcomes and safety events. PG-based dosing algorithms may ultimately be developed to optimize drug exposure and treatment outcomes.
Read more in the Genomics and Precision Health Blog.
Submitted by Carla Jeffries, JD, MPH, Wendy Carr, PhD, Ekaterina Kurbatova, MD, PhD, MPH, Meredith Moore, MPH, and Bria Marlowe, MPH, CHES

Think. Test. Treat TB Campaign
CDC is pleased to announce the Think. Test. Treat TB campaign, which aims to raise awareness about latent TB infection, risk, and the link between latent TB infection and TB disease by encouraging Asian Americans and their healthcare providers to:
- Think about the risk factors and talk about TB
- Test for TB infection
- Treat latent TB infection to prevent the development of TB disease
The Think. Test. Treat TB website has free resources in multiple languages, including TB messages, digital and print resources, social media content, patient and provider education materials, and a partner toolkit.
Here are some ideas to help spread the word:
- Post Test. Treat TB messages and use the hashtag #ThinkTestTreat on social media.
- Share Test. Treat TB digital content, video public service announcementexternal icon, and materials online.
- Use the sample articles to share Test. Treat TB information with communities and healthcare providers in newsletters, emails, and other partner communications.
- Download and distribute free education materials for patients and healthcare providers.
- Educate communities at risk and healthcare providers at meetings, health fairs, conferences, and other events.
For more information about this campaign, visit cdc.gov/thinktesttreattb and follow our activities on Twitter and Facebook.
Submitted by Leeanna Allen, MPH and Nick DeLuca, PhD

World TB Day Activities
On World TB Day, CDC Director Dr. Rochelle Walensky released a Dear Colleague Letter that highlighted the dedication of TB programs, public health departments, and healthcare facilities to prevent and treat TB during the COVID-19 pandemic. Dr. Walensky recognized the continued work and commitment by CDC and its partners, both domestically and globally, to eliminate TB in the United States and around the world.

CDC U.S. TB Elimination Champions
The 2022 CDC U.S. TB Elimination Champions marked the 7th year of recognizing the continued hard work and dedication from those working to end TB in their communities.
This year, CDC recognized organizations and individuals who have adapted to the shift in public health activities, developed innovative strategies to continue preventing and controlling TB in the United States, and played an important role in the COVID-19 response while continuing TB elimination activities.
Congratulations to the 2022 U.S. TB Elimination Champions:
- Breathe Easy South Texas (B.E.S.T.) Project – San Antonio, Texas
- Dr. Connie Haley, Medical Consultant and Comprehensive TB Clinical Course Co-Director, Southeastern National Tuberculosis Center
- Harris County Public Health Tuberculosis Program – Houston, Texas
- Amy Hill, RN, TB Program Manager, Oklahoma State Department of Health
- Martin Luther King Jr. Hospital, Infection Prevention Team – Los Angeles, California
- Monterey County Public Health Department TB Control Unit – Salinas, California
- Solano County Public Health Tuberculosis Control Program – Fairfield, California
- TB Elimination Alliance
World TB Day Events Timeline
To highlight World TB Day activities happening across the country, CDC created a 2022 World TB Day Events Timeline. Thank you to everyone who shared a World TB Day activity – there were 15 different events featured on the website! Learn more about the events that occurred across the United States on the Event Timeline webpage.
World TB Day Digital Media Toolkit
This year, CDC created a World TB Day digital toolkit designed to help plan communication activities to inform and educate partners, stakeholders, and media about TB-related problems and solutions, and the importance of supporting TB control and prevention efforts. The digital media toolkit included sample messaging, resources, and events.
Submitted by Kevin Crooks, MPH
Provisional TB Surveillance Data for 2021
On March 24, 2022, DTBE published provisional 2021 U.S. TB data in Morbidity and Mortality Weekly Report.
The COVID-19 pandemic has had a substantial effect on TB disease trends in the United States. Before COVID-19, TB disease diagnoses typically declined between 1% and 2% each year. However, data show that during COVID-19, reported TB disease diagnoses fell 20% in 2020 and remained 13% lower in 2021 than pre-pandemic levels. While these changes represent true reduction in TB disease, concerns remain about missed or delayed TB disease diagnoses.
CDC provides information and resources to help providers prevent, diagnose, and treat TB and is committed TB elimination. Healthcare and public health systems must be restored and strengthened to address TB disease in the wake of COVID-19, and prevent TB through expanded partnerships with providers and community-based organizations.
Submitted by Julie Self, PhD
Decrease in Tuberculosis Cases during COVID-19 Pandemic also Reflected by Outpatient Pharmacy Data
The COVID-19 pandemic has affected many areas of public health, including TB prevention and response. TB cases reported to the U.S. National TB Surveillance System in 2020 decreased 20% compared with the average number of cases reported during 2016–2019. Investigators at CDC analyzed outpatient pharmacy data, namely anti-TB medication dispensing, to determine the extent to which the reported decrease in TB cases was actual decline, a result of underreporting cases to public health (but persons with TB were still treated), or an indicator of missed or delayed TB diagnoses.
The outpatient pharmacy data also showed large declines in 2020, which strongly correlated with national TB surveillance case counts, helping to rule out underreporting as a cause. However, the strong possibility of underdiagnosis means that public health programs should be prepared for a possible rebound in TB cases after the pandemic, given that delayed and missed diagnoses could result in increased transmission because people with TB disease remain infectious for longer periods of time.
Read the full article in Emerging Infectious Diseases and hear more in a corresponding podcast.
Submitted by Kathryn Winglee, PhD
Surveillance definitions for extensively drug resistant (XDR) and pre-XDR tuberculosis
Effective with national TB products published starting this year (using data on TB cases counted in 2021), CDC is adopting new hybrid definitions of extensively drug-resistant (XDR) and pre-XDR TB for U.S. TB surveillance purposes. The hybrid definitions allow for either the existing U.S. definitions or the new 2021 WHO definitions (announced January 2021) to be used, with the exception that documented resistance to isoniazid in addition to rifampin (WHO definitions are inclusive of rifampin resistance only) would be a requirement for all U.S. resistance classifications. This approach keeps the current U.S. pre-XDR and XDR TB definitions intact but recognizes the revised WHO definitions within the United States and adds bedaquiline and linezolid to the list of drug classes that are considered when classifying cases into resistance groups. The new U.S. surveillance definitions are shown below:
- Multidrug-resistant (MDR) TB: caused by an organism that is resistant to at least isoniazid and rifampin
- Pre-XDR TB: caused by an organism that is resistant to isoniazid, rifampin, and a fluoroquinolone OR by an organism that is resistant to isoniazid, rifampin, and a second-line injectable (i.e., amikacin, capreomycin, and kanamycin)
- XDR TB: caused by an organism that is resistant to isoniazid, rifampin, a fluoroquinolone, and a second-line injectable (amikacin, capreomycin, and kanamycin) OR by an organism that is resistant to isoniazid, rifampin, a fluoroquinolone, and bedaquiline or linezolid
Read the Dear Colleague Letter for more information.
Submitted by Julie Self, PhD
Disease Surveillance Among U.S.-Bound Immigrants and Refugees — Electronic Disease Notification System, United States, 2014–2019
Each year, approximately 500,000 immigrants and tens of thousands of refugees (range: 12,000–85,000 during 2001–2020) move to the United States. While still abroad, immigrants, refugees, and others who apply for admission to live permanently in the United States must undergo a medical examination.
A new report published in MMWR summarizes health information that was reported to CDC’s Electronic Disease Notification (EDN) system for refugees, immigrants, and eligible others who arrived in the United States during 2014–2019.
The EDN system has both surveillance and programmatic components. The surveillance component is a centralized database that collects 1) health-related data from the overseas medical examination for immigrants with class A or B TB conditions and for all refugees and eligible others and 2) TB-related data from the post-arrival TB examination.
During 2014–2019, the overseas medical examination system prevented importation of 6,586 cases of infectious TB, 815 cases of syphilis, and 131 cases of gonorrhea. When the examination is used to offer public health interventions, most refugees (up to 96%) accept the intervention. Post-arrival follow-up examinations, which were completed for 88,190 persons and identified 475 cases of culture-positive TB, represent an important opportunity to further limit spread of TB disease in the United States by identifying and providing, if needed, preventive care for those with latent TB infection or treatment for those with TB disease.
Additional public health interventions that could be offered during the overseas medical examination should be considered (e.g., treatment for latent TB infection). Finally, for persons with class B TB, measures should be taken to identify and remove barriers to completing post-arrival examinations to reduce risk for TB disease and community transmission, along with measures to encourage reporting of completed examinations for better data-driven decision-making.
Read the full report in MMWR published on January 21, 2022. For additional background information, visit the EDN webpage.
Submitted by Julie Self, PhD

Provision Guidance for the Use of BPaL
In February, CDC released “Provisional CDC Guidance for the Use of Pretomanid as part of a Regimen [Bedaquiline, Pretomanid, and Linezolid (BPaL)] to Treat Drug-Resistant Tuberculosis Disease.” The provisional guidance includes considerations for clinicians, dosing and administration, precautions and adverse event monitoring, microbiologic monitoring, and more.
CDC encourages clinicians, pharmacists, and public health professionals to review the provisional guidance. Clinicians can contact state and local TB control offices and the TB Centers of Excellence for Training, Education, and Medical Consultation for additional information on diagnosing and treating drug-resistant TB disease.
Submitted by Terence Chorba, MD, MPH, DSc and John Parmer, PhD
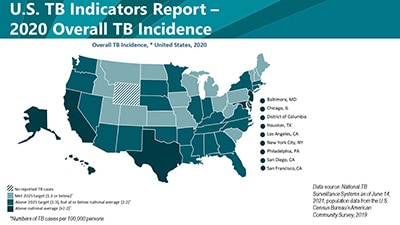
2020 State and City Tuberculosis Report
In January, CDC published the 2020 State and City Tuberculosis Report . This report provides key process and outcomes measures for TB control programs in the United States. These measures are used to monitor progress towards TB elimination at the state and local level. The 2020 report contains a new section on health equity goals for populations of people who experience health disparities related to TB disease. Data for calculating these measurement indicators are derived from the National Tuberculosis Surveillance System (NTSS) and the Aggregate Reports for Program Evaluation (ARPE). CDC publishes indicator data to assist TB programs in:
- Evaluating progress toward achievement of national objectives through monitoring of TB program performance.
- Assessing the need for education and technical assistance.
- Identifying areas that need improvement.
Learn more about the National TB Program Objectives and Performance Targets for 2025.
Submitted by Rachel Yelk Woodruff, MPH and Bria Marlowe, MPH, CHES
Laboratory Branch Welcomes New Laboratory Scientists
Wan Moon joins DTBE’s Laboratory Branch as a Quality Manager supporting the branch laboratory quality management system and accreditation efforts. Before working at CDC, Wan worked in the Molecular/Emergency Preparedness Branch at the Georgia Public Health Laboratory and as a Senior Microbiologist at the Texas Department of State Health Services in the Serological Analysis/HIV/STD laboratory. Wan obtained his BS in Biology from Texas A&M University.
Ashlie Blinn is the newest biologist on the Reference Laboratory Team where she performs CLIA-compliant growth-based drug susceptibility testing to provide critical results to help guide treatment decisions for patient care. Prior to joining CDC, Ashlie served as an Environmental Health Technician/Science Officer in the United States Army and as a Biology Instructor and Science Laboratory Coordinator at West Georgia Technical College. Ashlie holds an MS and BS in Biology from University of West Georgia.
Sean Buono is the newest Laboratory Consultant on the Laboratory Capacity Team providing oversight and technical consultation to the laboratory component of the TB Cooperative Agreement. Sean began his CDC career in the Meningitis and Vaccine Preventable Disease Branch, Bacterial Meningitis Laboratory as a CDC Laboratory Leadership Service fellow. Sean also worked in the Public Health Microbiologist Research and Training Unit at the Los Angeles County Public Health Laboratory and at the San Francisco Public Health Laboratory as an Emerging Infectious Disease Training fellow. Sean obtained his PhD in Environmental Health Sciences at UCLA Fielding School of Public Health and his BS in Science from University of California Irvine.
Wenming (Ming) Zhu joins the Laboratory Branch as a microbiologist on the Applied Research Team performing research improving understanding and detection of drug-resistance in Mycobacterium tuberculosis. Prior to joining the Laboratory Branch, Ming was a member of the CDC Clinical and Environmental Microbiology Branch in the Division of Healthcare Quality Promotion. Ming completed his postdoctoral training at Emory University, received his PhD in Molecular Microbiology from University of Mississippi Medical Center and his MS and BS in Laboratory Medicine and Microbiology from Third Medical College in Chongqing, China.

Newly Released Tuberculosis Laboratory Aggregate Report
DTBE’s Laboratory Branch recently published the TB Laboratory Aggregate Report—Sixth Editionpdf icon. This report includes a comparison of cooperative agreement awardee aggregate workload volume data, nucleic acid amplification test (NAAT) trends, and workload performance turnaround time (TAT) data for calendar years 2017, 2018, and 2019. Also included in this report is the most recent information (2020) regarding TB testing methods in awardee public health laboratories (PHLs). This report is compiled for PHLs to assess progress towards meeting national TB testing benchmarks and for peer comparison with other PHLs with similar specimen or testing volume, using similar methods, or in a similar geographical location.
Submitted by Stephanie Johnston, MS and Monica Youngblood, MPH

TB Elimination Alliances Releases Strategic Plan
The TB Elimination Allianceexternal icon has released their 2021 and 2022 Strategic Planpdf iconexternal icon that includes information on community engagement, access to testing and treatment, provider education, as well as research and data.
Burzynski J, Mangan JM, Lam CK, Macaraig M, Salerno MM, deCastro BR, Goswami ND, Lin CY, Schluger NW, Vernon A; eDOT Study Team. In-person vs electronic directly observed therapy for tuberculosis treatment adherence: a randomized noninferiority trial. JAMA Netw Open 2022;5(1):e2144210. (Lam, also with the New York City Department of Health).
DeCuir J, Baggs J, Melgar M, Patel P, Wong KK, Schwartz NG, Bamrah Morris S, Godfred-Cato S, Belay ED. Identification and description of patients with multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection using the Premier Healthcare Database. Epidemiol Infect 2022. Epub ahead of print.
Ho CS, Feng PJ, Narita M, Stout JE, Chen M, Pascopella L, Garfein R, Reves R, Katz DJ; Tuberculosis Epidemiologic Studies Consortium. Discordant results of tests for tuberculosis reconsidered—authors’ reply. Lancet Infect Dis 2022;22(2):164–5.
Katrak SS, Li R, Reynolds S, Marks SM, Probst JR, Chorba T, Winthrop K, Castro KG, Goswami ND. Association of tumor necrosis factor α inhibitor use with diagnostic features and mortality of tuberculosis in the United States, 2010–2017. Open Forum Infect Dis 2021;9(2):ofab641. (Goswami, also with Emory University).
Lambrou AS, Shirk P, Steele MK, Paul P, Paden CR, Cadwell B, Reese HE, Auki Y, Hassell N, Zheng X, Talarico S, Chen JC, Oberste MS, Batra D, McMullan LK, Laufer Halpin A, Galloway SE, MacCannell DR, Kndor R, Barnes J, MacNeil A, Silk BJ, Dugan VG, Scobie HM, Wentworth DE; Strain Surveillance and Emerging Variants Bioinformatic Working Group; Strain Surveillance and Emerging Variants NS3 Working Group. Genomic surveillance for SARS-CoV-2 variants: predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) variants—United States, June 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:206–11.
Nelson KN, Talarico S, Poonja S, McDaniel CJ, Cilnis M, Chang AH, Raz K, Noboa WS, Cowan L, Shaw T, Posey J, Silk BJ. Mutation of Mycobacterium tuberculosis and implications for using whole-genome sequencing for investigating recent tuberculosis transmission. Front Public Health 2022;9:790544.
Phares CR, Liu Y, Wang Z, Posey DL, Lee D, Jentes ES, Weinberg M, Mitchell T, Stauffer W, Self JL, Marano N. Disease surveillance among U.S.-bound immigrants and refugees—Electronic Disease Notification system, United States, 2014–2019. MMWR Surveill Summ 2022;71(No. SS-2):1–21.
Shrestha S, Winglee K, Hill A, Shaw T, Smith J, Kammerer JS, Silk BJ, Marks S, Dowdy D. Model-based analysis of tuberculosis genotype clusters in the United States reveals high degree of heterogeneity in transmission, and state-level differences across California, Florida, New York, and Texas. Clin Infect Dis 2022. Epub ahead of print.
Wang Z, Posey DL, Brostrom RJ, Morris SB, Marano N, Phares CR. Post-arrival evaluation of immigrant and refugee children in the USA with latent tuberculosis infection diagnosed overseas, 2007‒2019. J Pediatr 2022:S0022-3476(22)00080-4. Epub ahead of print.
Liu Y, Posey DL, Yang Q, Weinberg MS, Maloney SA, Lambert LA, Ortega LS, Marano N, Cetron MS, Phares CR. Multidrug-resistant tuberculosis in U.S.-bound immigrants and refugees. Ann Am Thorac Soc 2021. Epub ahead of print.
Podany AT, Pham M, Sizemore E, Martinson N, Samaneka W, Mohapi L, Badal-Faesen S, Dawson R, Johnson JL, Mayanja H, Lalloo U, Whitworth WC, Pettit A, Campbell K, Phillips P, Bryant K, Scott N, Vernon A, Kurbatova E, Chaisson RE, Dorman S, Nahid P, Swindells S, Dooley KE, Fletcher CV; AIDS Clinical Trials Group & Tuberculosis Trials Consortium. Efavirenz pharmacokinetics and HIV-1 viral suppression among patients receiving TB treatment containing daily high-dose rifapentine. Clin Infect Dis 2021. Epub ahead of print.
Smith JP, Gandhi NR, Silk BJ, Cohen T, Lopman B, Raz K, Winglee K, Kammerer S, Benkeser D, Kramer M, Hill AN. A cluster-based method to quantify individual heterogeneity in tuberculosis transmission. Epidemiology 2021. Epub ahead of print.
Wanga V, Gerdes ME, Shi DS, Choudhary R, Dulski TM, Hsu S, Idubor OI, Webber BJ, Wendel AM, Agathis NT, Anderson K, Boyles T, Chiu SK, Click ES, Da Silva J, Dupont H, Evans M, Gold JAW, Haston J, Logan P, Maloney S, Martinez M, Natarajan P, Spicer KB, Swancutt M, Stevens V, Brown J, Chandra G, Light M, Barr FE, Snowden J, Kociolek LK, McHugh M, Wessel D, Simpson JN, Gorman KC, Breslin, KA, DeBiasi RL, Thompson A, Kline MW, Bloom JA, Singh IR, Dowlin M, Wietecha M, Schweitzer B, Bamrah Morris S, Koumans EH, Ko JY, Kimball AA, Siegel DA. Characteristics and clinical outcomes of children and adolescents aged <18 years hospitalized with COVID-19—six hospitals, United States, July–August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1766–72. (Also from DVH [Brown] and DHP [Kimball]).
Belay ED, Godfred Cato S, Rao AK, Abrams J, Wilson WW, Lim S, Newton-Cheh C, Melgar M, DeCuir J, Webb B, Marquez P, Su JR, Meng L, Grome HN, Schlaudecker E, Talaat K, Edwards K, Barnett E, Campbell AP, Broder KR, Bamrah Morris S. Multisystem inflammatory syndrome in adults after SARS-CoV-2 infection and COVID-19 vaccination. Clin Infect Dis 2021. Epub ahead of print.
Fast HE, Zell E, Patel Murthy B, Murthy N, Meng L, Gibbs Scharf L, Black CL, Shaw L, Chorba T, Harris LQ. Booster and additional primary dose COVID-19 vaccinations among adults aged ≥65 years—United States, August 13, 2021–November 19, 2021. MMWR Morb Mortal Wkly Rep 2021;70(50):1735–9.
Ghosh S, Felix D, Kammerer JS, Talarico S, Brostrom R, Starks A, Silk B. Evaluation of sputum-culture results for tuberculosis patients in the United States-Affiliated Pacific Islands. Asia Pac J Public Health 2021. Epub ahead of print.
Kaniga K, Hasan R, Jou R, Vasiliauskienė E, Chuchottaworn C, Ismail N, Metchock B, Miliauskas S, Viet Nhung N, Rodrigues C, Shin S, Simsek H, Smithtikarn S, Ngoc ALT, Boonyasopun J, Kazi M, Kim S, Kamolwat P, Musteikiene G, Sacopon CA, Tahseen S, Vasiliauskaitė L, Wu MH, Vally Omar S. Bedaquiline Drug Resistance Emergence Assessment in MDR-TB (DREAM): a 5-year prospective in-vitro surveillance study of bedaquiline and other second-line drug-susceptibility testing in MDR-TB isolates. J Clin Microbiol 2021. Epub ahead of print.
Nabity SA, Han E, Lowenthal P, Henry H, Okoye N, Chakrabarty M, Chitnis AS, Kadakia A, Villarino E, Low J, Higashi J, Barry PM, Jain S, Flood J. Sociodemographic characteristics, comorbidities, and mortality among persons diagnosed with tuberculosis and COVID-19 in close succession in California, 2020. JAMA Netw Open 2021;4(12):e2136853. (Nabity, also with California Department of Public Health).
Njie GJ, Khan A. Prevalence of tuberculosis and mental disorders comorbidity: a systematic review and meta-analysis. J Immigr Minor Health 2021. Epub ahead of print.
Punetha A, Green KD, Garzan A, Thamban Chandrika N, Willby MJ, Pang AH, Hou C, Holbrook SYL, Krieger K, Posey JE, Parish T, Tsodikov OV, Garneau-Tsodikova S. Structure-based design of haloperidol analogues as inhibitors of acetyltransferase Eis from Mycobacterium tuberculosis to overcome kanamycin resistance. RSC Med Chem 2021;12(11):1894–909.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Kevin Crooks at qyd7@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.