Tuberculosis Trials Consortium (TBTC)

CDC assesses the need for and conducts studies of new or existing drugs and regimens used in the prevention and treatment of TB, including dosage, duration, pharmacokinetics, and toxicity. Currently, most of these trials and studies are conducted by the Tuberculosis Trials Consortium (TBTC), a unique collaboration of researchers from CDC, domestic and international public health departments and academic medical centers, and selected Veterans Administration medical centers. TBTC’s mission is to conduct programmatically relevant research concerning the diagnosis, clinical management, and prevention of tuberculosis infection and disease.
The work of TBTC:
- expands clinical and epidemiologic knowledge of TB,
- integrates research into the care of persons with TB infection and disease,
- promotes research within local TB programs through collaboration on clinical research of relevance to public health settings, and
- provides a forum for international collaborative research of importance to both domestic and international TB control.
Since its inception, TBTC clinical trials have had significant impact on TB treatment and prevention:
- U.S. Public Health Service Study 22 substantially influenced American Thoracic Society (ATS)/CDC/Infectious Disease Society of America (IDSA) guidelines for treatment of TB disease. TBTC Study 34 influenced the 2017 ATS/CDC/IDSA guidelines for TB diagnosis, and TBTC Studies 26 and 33 led to updates in National Tuberculosis Controllers Association/CDC guidelines for treatment of latent TB infection.
- TBTC Study 31/ACTG A5349 was the first trial to achieve success with a 4-month treatment regimen for active TB; it demonstrated that a four-month daily treatment regimen containing a combination of high-dose rifapentine and moxifloxacin is non-inferior to the standard six-month daily regimen for drug-susceptible TB disease. The results of this study will inform future TB disease treatment guidelines and will guide future treatment-shortening trials.
- TBTC Study 23 results have led to modification of CDC’s recommendations for treatment of TB and HIV.
The current pipeline of new and re-purposed anti-TB drug candidates is the most promising in 40 years. Advances in TB clinical trials science have fostered the progress of these agents, supported by many members of the global TB control community. With commitment and support from CDC, the TBTC adds importantly to the resources available for these clinical trials and is expected to continue to make useful contributions to TB treatment, prevention, and control.
Activities of TBTC include:
- TBTC Study 31: this large trial of a 4-month rifapentine-based regimen for treatment of drug-sensitive TB, conducted in collaboration with the National Institute of Allergy and Infectious Diseases, AIDS Clinical Trials Group (as ACTG A5349), demonstrated non-inferiority of one of two test regimens. The trial has concluded, and many analytic activities are currently underway. ClinicalTrials.gov Identifier: NCT02410772
- TBTC Study 35: this is a Phase I/2 open-label, single arm, exposure-controlled study to determine appropriate dosing of a novel water-dispersible, child-friendly formulation of rifapentine with isoniazid in children aged 0-12 years. The trial aims also to assess safety of this formulation in HIV-infected and HIV-uninfected children. If successful, the trial will contribute to global availability of a pediatric formulation that can be used to treat latent TB infection in young children. ClinicalTrials.gov Identifier: NCT03730181
- TBTC Study 37: this is an open label, multi-center, phase 3 randomized controlled non-inferiority trial that compares the safety and effectiveness of a 6-week regimen of daily rifapentine against the current standard of 12-16 weeks of rifamycin-based treatment for latent TB infection. ClinicalTrials.gov Identifier: NCT03474029
- CRUSH-TB: this phase 2C trial aims to assess the efficacy and safety of several regimens based on novel agents in the treatment of drug-sensitive TB disease. The trial is well-advanced in planning.
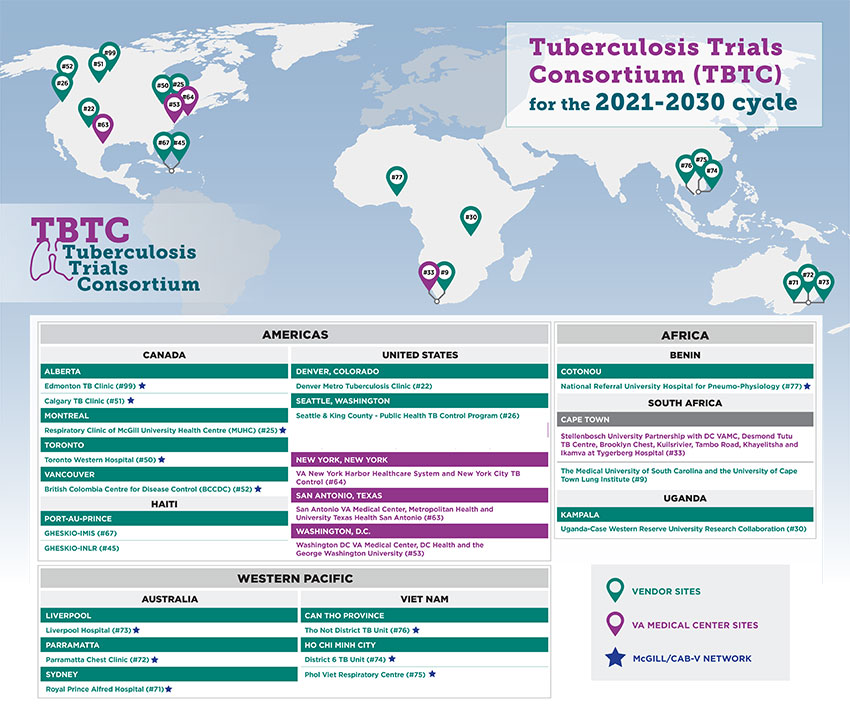
TBTC sites are funded in one of two ways, either through individual contracts with CDC or as part of a sub-network coordinated by investigators at the Washington, D.C. Veterans Affairs (VA) Medical Center. Every 10 years, both sides of the consortium undergo re-competition. In March 2021, CDC announced the sites for the research cycle through December 2030.
As in prior years, the TBTC re-competition resulted in awards to new as well as previous clinical sites. The members of TBTC combine outstanding scientific expertise and demonstrated TB trials capacity. As in the past, some of these awards reflect collaborations involving North American and other international institutions.
TBTC sites are located in Australia, Benin, Canada, Haiti, South Africa, Uganda, the United States, and Vietnam.
Developing new TB treatment and prevention strategies depends upon collaboration among academics, private sector and government researchers, public health departments, manufacturers of pharmaceuticals, regulatory agencies, and non-governmental organizations. CDC works closely with such organizations as:
- the U.S. Food and Drug Administration,
- the National Institutes of Health and its National Institute of Allergy and Infectious Diseases,
- the Global Alliance for TB Drug Development, and
- others within and outside the United States.
Such partnerships build upon the long tradition of collaboration in pursuit of important public health goals.
CDC
The CDC oversees, and collaborates in, the work of TBTC. The TBTC CDC team is composed of medical officers, epidemiologists, trialists, health scientists, microbiologists, data analysts, data managers, programmers, data clerks, multiple public health students, administrative support staff, and others. The CDC team supports the TBTC by:
- functioning as the TBTC’s Data and Coordinating Center;
- participating in protocol development;
- guiding implementation, analysis, and interpretation of each trial;
- conducting the central monitoring for all TBTC trials;
- performing data management;
- managing drug supplies;
- establishing site laboratory standards;
- executing pharmacovigilance for TBTC trials;
- presenting scientific data at conferences and in author publications; and
- coordinating administrative, regulatory, and fiscal support for the TBTC.
TBTC Committees
Several working committees make up the governance of TBTC:
- The Steering Committee, made of representatives from all those engaged, makes major decisions for the group.
- The Executive Affairs Group serves as the executive arm of the Steering Committee and conducts the Consortium’s day-to-day administrative business.
- The Core Science Group develops the scientific program of research.
- The Implementation and Quality Committee supervises the conduct and quality of ongoing studies.
- The Publications and Presentations Committee assures quality and equity in TBTC’s reporting of its work.
- The Advocacy and External Relations Committee represents the TBTC to outside entities, and supports the Consortium’s community engagement activities.
CDC staff are represented and participate on all committees.