TB NOTES

TB Notes 2, 2021
Notes from the Director

Dear Colleague,
This spring has been a busy season for the Division of Tuberculosis Elimination (DTBE) staff. Many DTBE staff members and our TB colleagues across the country continue to address public health needs in the response to the COVID-19 pandemic. As of June 21, 2021, a total of 114 DTBE staff members (72% of DTBE staff) have participated in a cumulative of 284 deployments supporting CDC’s COVID-19 response efforts. A total of 66 DTBE staff members have participated in multiple deployments. Thank you to everyone who has responded to the COVID-19 pandemic.
On May 13, 2021, the White House announcedexternal icon a $1.13 billion investment to strengthen the disease intervention specialists (DIS) workforce. This funding is from the American Rescue Plan Act of 2021 and will be used over a five-year period to support DIS and DIS-related training and retention, and related technological advances to address COVID-19 and other infectious diseases, including TB, across the nation. Learn more in a Dear Colleague Letter from CDC’s Division of STD Prevention.
While public health professionals across the United States continue to provide support to the COVID-19 Emergency Response, critical work to eliminate TB disease in the United States also continues. On May 6, 2021, The New England Journal of Medicine published “Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosisexternal icon,” which highlights findings from Study 31/A5349, a collaboration of CDC’s Tuberculosis Trials Consortium (TBTC) and the National Institute of Health’s Adult AIDS Clinical Trials Group (ACTG). Study 31/A5349 is the first clinical trial to identify a shorter 4-month daily treatment regimen for drug-susceptible tuberculosis (TB) disease that is as effective as (non-inferior to) the existing 6-month daily regimen in curing TB disease. This is the first new treatment regimen for drug-susceptible TB disease in almost 40 years. I am grateful to the researchers, clinical staff, and most of all, study participants, for their important contributions to this study.
DTBE also announced the Tuberculosis Trials Consortium (TBTC) for the 2021-2030 cycle. CDC awarded contracts for the next ten-year research cycle, initiating the 28th year of the TBTC collaborative effort. The new and continuing members of TBTC combine outstanding scientific expertise and superb TB trials capacity. This exciting collaboration will continue to contribute to development of stronger approaches to the prevention and treatment of TB over the next decade. More information about the new contract, including the awardees, is provided in a recent Dear Colleague Letter. The awardees were able to meet during TBTC’s 47th semi-annual meeting held as a virtual meeting June 7-8, 2021.
Recently, DTBE’s Reference Laboratory Team Lead Beverly Metchock, Dr.P.H., D (ABMM), was named the 2021 Association of Public Health Laboratories (APHL) “On the Front Line” award winnerexternal icon. The award honors an individual or laboratory staff outside of the APHL membership that makes significant contributions to the advancement of public health laboratory science and/or practice. I would like to congratulate Dr. Metchock on this well-deserved recognition.
Finally, the 2018 Contact Investigation Report was published in April. The report summarizes national contact investigation efforts related to TB cases diagnosed in 2018, along with summary data from 2014-2017. The data are derived from the Aggregates Reports for Program Evaluation (ARPE) and Report of Verified Case of TB surveillance system. DTBE encourages each program to review its contact investigation results and compare them to the 2025 national performance targets and the national averages available through the National Tuberculosis Indicators Project. Programs are encouraged to identify barriers to their contact investigation processes and to design and implement strategies to overcome these barriers and improve contact investigation performance.
As we enter the second half of the year, I continue to be inspired by your dedication to the very important work you do every day in our efforts to eliminate TB. I hope all of you have a safe, enjoyable, and productive summer.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
New England Journal of Medicine: TB Trial Identifies Shorter Four-Month Treatment Regimen
CDC’s Tuberculosis Trials Consortium (TBTC), together with collaborators from the National Institutes of Health’s (NIH) AIDS Clinical Trial Group (ACTG), published “Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis”external icon in the New England Journal of Medicine. The article is accompanied by a supplementexternal icon, editorialexternal icon, videoexternal icon and Research Summaryexternal icon.
The article details findings from Study 31/A5349 – an international, randomized, controlled, open label, phase 3 non-inferiority clinical trial. The trial demonstrated that a shorter four-month daily treatment regimen with high-dose (“optimized”) rifapentine and moxifloxacin is as effective as (non-inferior to) the standard daily six-month regimen in curing drug-susceptible TB disease. This important study was also presented during the virtual 51st Union World Conference on Lung Health.
Study 31/A5349 examined the efficacy and safety of two four-month regimens with high-dose rifapentine with or without moxifloxacin for the treatment of drug-susceptible TB disease.
- The successful four-month regimen – 2PHZM/2PHM – included eight weeks of daily treatment with rifapentine (P), isoniazid (H), pyrazinamide (Z), and moxifloxacin (M) and nine weeks of daily treatment with rifapentine (P), isoniazid (H), and moxifloxacin (M). At the conclusion of the trial, the four-month regimen met non-inferiority criteria for efficacy in all analyses and was safe and well-tolerated.
- A second four-month regimen – 2PHZE/2PH – included eight weeks of daily treatment with rifapentine (P), isoniazid (H), pyrazinamide (Z), and ethambutol (E) and nine weeks of daily treatment with rifapentine (P) and isoniazid (H). This new regimen did not meet non-inferiority criteria when compared to the existing standard regimen.
- The existing six-month regimen –2RHZE/4RH – includes eight weeks of daily treatment with rifampin (R), isoniazid (H), pyrazinamide (Z), and ethambutol (E) and approximately 18 weeks of daily treatment with rifampin (R) and isoniazid (H).
This is the first successful short treatment regimen for drug-susceptible TB disease identified in almost 40 years. The availability of shorter regimens enables patients to be cured faster, and has the potential to reduce treatment costs, improve patient quality of life, and increase completion of therapy.
CDC will use the results of this study to help inform future TB disease treatment guidelines.
Submitted by Philip LoBue, MD, FACP, FCCP, Division Director
Tuberculosis Trials Consortium Awards for the 2021-2030 Cycle
DTBE conducts vital, unparalleled clinical trials and epidemiologic research through the Tuberculosis Trials Consortium (TBTC), which advances the TB elimination strategy in the United States and globally. Earlier this fiscal year, CDC awarded contracts for the next ten-year research cycle, 2021 – 2030, initiating the 28th year of this collaborative effort.
Since it began in 1993, TBTC has been responsible for several major clinical trials that have significantly impacted TB treatment. The new and continuing members of TBTC combine outstanding scientific expertise and superb TB trials capacity. Learn more about the TBTC awards for the 2021-2030 cycle in a recent Dear Colleague Letter.
Submitted by Philip LoBue, MD, FACP, FCCP, Division Director

Tuberculosis Trial Consortium 47th Semi-Annual Meeting
The 47th semi-annual Tuberculosis Trials Consortium (TBTC) meeting was held June 7-8, 2021 as a virtual meeting. The meeting was held on the Zoom platform and was a great success with approximately 160 participants worldwide. The agenda included a Director’s update on DTBE from Dr. LoBue; updates on studies 31, 35, 37, and CRUSH; special topics discussions; and priority setting for TBTC 2020-2029. This meeting also served as an introductory meeting for the new TBTC Consortium members. CRB is grateful to all who presented in this virtual forum and pleased to be able to successfully uphold the semi-annual meeting in the COVID-19 environment.
Submitted by Carla Jeffries, JD, MPH, Deputy Branch Chief

New “Introduction to Tuberculosis Slide Set” Now Available for Download
DTBE released a new slide set titled “Introduction to Tuberculosis (TB).” This slide set is meant to be a tool for people who are not familiar with TB. It provides a basic overview of TB using plain language and visual aids. Unlike the other DTBE slide sets which are often directed at those working in TB, this slide set is designed for a public audience.
The product is currently available online and can be downloaded from the CDC DTBE website.
We encourage you to use these slides to help educate your community and others about TB.
Submitted by Beth Bouwkamp, MPH, BA, ORISE Fellow

“Core Curriculum on TB” – Print Copies Now Available for Order
DTBE is pleased to announce that print copies of the updated Core Curriculum on Tuberculosis: What the Clinician Should Know are now available for order from CDC-INFO On Demand – Publications.
For information on how to order print copies of the guide, please see the publication ordering instructions. Requests for orders greater than 100 copies will need approval prior to shipping. The product is also available online and can be downloaded from the CDC DTBE website.
Continuing Education (CE) credits are available free of charge for completion of this educational activity. More information on continuing education can be found on the Core Curriculum CE webpage.
This document is intended for use as a reference manual for clinicians caring for persons with or at high risk for TB disease or infection.
Submitted by Beth Bouwkamp, MPH, BA, ORISE Fellow
DocStyles and Estilos Surveys: Using Audience Research to Understand Physician Practices for Latent Tuberculosis (TB) Infection Testing and Treatment and Knowledge and Perceptions of TB Disease Among Hispanic Adults in the United States
When planning interventions and communications campaigns, it is essential to understand your target audience. Audience research allows you to understand and get to know your intended target audience. CDC’s Office of the Associate Director for Communication provides technical assistance and support to CDC programs for their audience research needs, including access to multiple market research databases. One of these market research databases is the Porter Novelli Stylesexternal icon survey data sets. For the last 15 years, CDC has licensed data from Porter Novelli that provide insight into various population segments, including adults, youth, and health professionals. DTBE added questions about TB disease to the DocStyles and Estilos surveys conducted in fall 2020.
DocStyles is a web-based survey that is currently fielded twice a year in the spring and fall. Quotas are set to reach 1,000 primary care physicians (family practitioners and internists) and 250 of each specialty audience. The survey contains 135 questions, some with multiple subparts, which were designed to provide insight into health care providers’ attitudes and counseling behaviors in regard to a variety of health issues and to assess their use of available health information sources. Two questions related to TB disease were added for the first time in fall 2020. The first TB question was “Do you routinely test non-U.S. born patients for tuberculosis (TB)?” The second TB question was “Which treatment regimens for latent TB infection do you prescribe?” In addition to survey answers, data is collected on the state the practitioner works in, number of years practicing medicine, and medical practice specialty.
Estilos is the Spanish name for Styles and is a survey that reaches about 1000 Hispanic adults. Information regarding language preference, years spent in-country, and cultural identification are captured to ensure adequate representation from Hispanics of various acculturation levels. Two TB- related questions were also added to this survey. The first question was “Have you ever been tested or vaccinated for tuberculosis (TB)?” The second question was “What are your chances of getting tuberculosis (TB)?”
The results of the DocStyles and Estilos surveys provide insight into TB topics related to doctors and Spanish speaking communities. DocStyles results tables below show responses based on medical specialty.
Physician DocStyles Question 1: Do you routinely test non-U.S. born patients for tuberculosis (TB)?
| Yes- Skin Test | Yes- Blood Test | Yes- Skin and Blood | Do not do regular testing | Refer to Health Department | Prefer not to answer | |
|---|---|---|---|---|---|---|
| Total | 26% | 17% | 14% | 36% | 6% | 1% |
| PCP (n=1000) | 24% | 18% | 14% | 38% | 5% | 1% |
| Pediatricians (n=252) | 35% | 16% | 19% | 24% | 5% | 0% |
| Ob/Gyn (n=0) | 0% | 0% | 0% | 0% | 0% | 0% |
| NP/NA (n=251) | 26% | 15% | 9% | 38% | 11% | 2% |
Physician DocStyles Question 2: Which treatment regimens for latent tuberculosis infection (LTBI) do you prescribe?
| 3mos INH + Rifapentine | 4mos Rifampin | 3mos INH + Rifampin | 6mos INH | 9mos INH | Refer to Health Dept | None of these | |
|---|---|---|---|---|---|---|---|
| Total (n=1503) | 10% | 9% | 21% | 17% | 27% | 37% | 4% |
| PCP (n=1000) | 11% | 11% | 24% | 21% | 29% | 31% | 4% |
| Pediatricians (n=252) | 9% | 6% | 16% | 12% | 37% | 36% | 3% |
| Ob/Gyn (n=0) | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| NP/ NA (n=251) | 7% | 5% | 17% | 8% | 9% | 63% | 7% |
Estilos results tables below show responses based on overall responses.
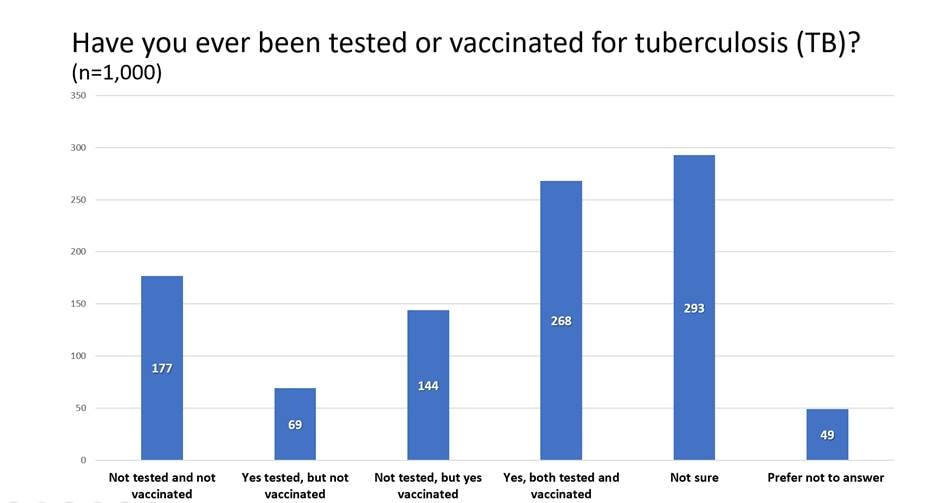
Hispanic Adults Estilos Question 1: Have you ever been tested or vaccinated for tuberculosis (TB)?
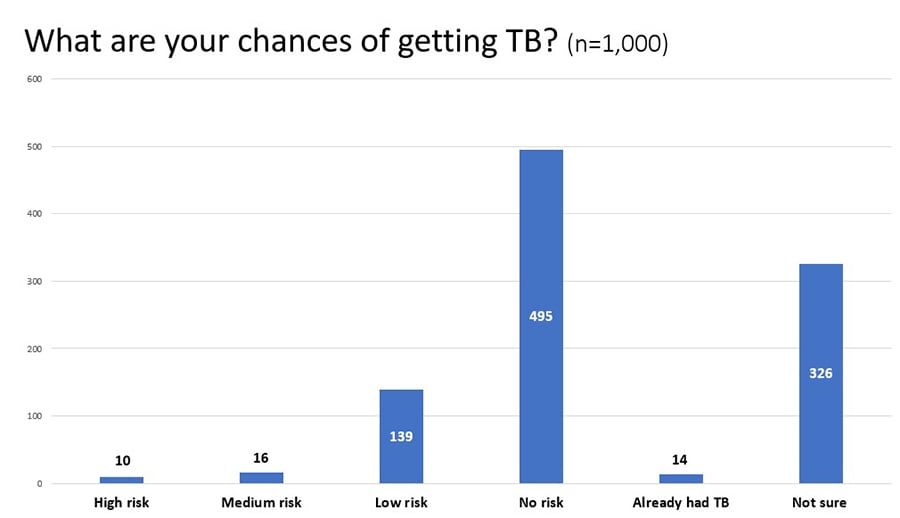
Hispanic Adults Estilos Question 2: What are your chances of getting tuberculosis (TB)?
DTBE plans to repeat the same questions on the DocStyles and Estilos surveys next year to compare the results. The results of the DocStyles and Estilos surveys will be applied to the division’s TB communication, education, and behavioral science work. DTBE will use the data to segment audiences based on key characteristics, to develop appropriate creative messaging, and evaluate message reach and recall among the intended audience. Specifically, DocStyles results will be used to guide development of continuing education resources and other engagement activities aimed at health care providers to improve use of IGRAs for testing and the use of short course regimens for the treatment of latent TB infection. Testing for TB infection should be a routine and integral part of health care for patients with increased risk for TB. Health care providers are encouraged to use newer TB blood tests to screen for TB infection. CDC and the National Tuberculosis Controllers Association (NTCA) preferentially recommend short-course, rifamycin-based, 3- or 4-month latent TB infection treatment regimens over 6- or 9-month isoniazid monotherapy (6H or 9H, respectively). Estilos results will be used to guide the development of communication messages and patient education materials for Hispanic adults.
Submitted by Beth Bouwkamp, MPH, BA, ORISE Fellow

Updated: TB Laboratory Infographics
CDC recently published three updated infographics to inform and educate partners on the work of the DTBE Laboratory. The infographics include Under the Microscope: A Closer Look at CDC’s Tuberculosis Laboratory, CDC’s Molecular Detection of Drug Resistance Service: Rapid Test Results for Real-Time Patient Care, and Whole Genome Sequencing (WGS) – New Lab Technology Helps Fight an Old Disease. The infographics are available for download on the CDC website.
Submitted by Leeanna Allen, MPH, Communications Team Lea

Surveillance, Epidemiology, and Outbreak Investigations Branch (SEOIB) Welcomes New Deputy Branch Chief
In January 2021, SEOIB welcomed Justin Davis as its new deputy branch chief. Justin was appointed acting deputy in August 2020. A CDC employee for more than 7 years, Justin brings a well-rounded set of skills applicable to both the administrative and programmatic functions of the Deputy Branch Chief role. Previously, as a policy analyst in DTBE’s Office of the Director, he co-developed and launched the CDC-managed TB drug stockpile, formulated annual domestic TB congressional budget justifications, led the development of a cooperative agreement focused on expanding latent TB testing and treatment, and handled responses to domestic TB-related program inquiries from internal and external stakeholders. He also served on the leadership committee responsible for writing the 2020-2025 TB cooperative agreement. Although most of his time at CDC has been with DTBE, Justin also worked for CDC’s Division of Unintentional Injury Prevention, where he played an important role in the expansion of CDC’s opioid overdose prevention efforts, including serving as lead project officer for the Enhanced State Opioid Overdose Surveillance program. He holds an MPH degree from Georgia State University and a Bachelor of Business Administration degree from Emory University.
Submitted by Adam Langer, DVM, MPH, DACVPM, Branch Chief
Laboratory Branch and Surveillance, Epidemiology, and Outbreak Investigations Branch Debut Whole-genome Multi-locus Sequence Typing for TB Cluster Evaluation
DTBE’s Laboratory Branch and Surveillance, Epidemiology, and Outbreak Investigations Branch will soon introduce a new genotyping technique that will assist TB programs with cluster investigations.
Whole-genome sequencing data has been routinely used since 2016 for whole-genome single nucleotide polymorphisms (SNP) comparisons to identify differences between isolates in a GENType cluster. The variations are mapped onto phylogenetic trees and can improve TB programs’ ability to identify cases that may be involved in recent transmission and rule out isolates unlikely to represent recent transmission. Combined with available epidemiologic and clinical data, TB programs may then focus their resources on cluster investigations that have the greatest potential to benefit individual patients and the larger community.
Whole-genome multi-locus sequence typing (wgMLST) also uses whole-genome sequencing of the Mycobacterium tuberculosis genome and determines the sequence of 2,700 individual genetic loci, producing a wgMLST pattern which is then given a wgMLSType name. Currently, TB control programs rely upon conventional genotyping methods (spoligotyping and 24-locus MIRU-VNTR (mycobacterial interspersed repetitive units – variable number of tandem repeats) combined to assign a GENType) to cluster related isolates. While the conventional methods compare ~1% of the M. tuberculosis genome, the wgMLST method compares 70% of the genome. The wgMLST approach of identifying clustered isolates will provide TB control programs with more precisely defined clusters and provide greater efficiency in the genotyping lab by eliminating the need for spoligotyping and MIRU-VNTR.
Beginning in June 2021, CDC’s TB Genotyping Information Management System (TB GIMS), a secure web-based system available to TB programs for dissemination and understanding of genotyping results and reports, will include wgMLSType for all isolates that were sequenced since the beginning of 2018 at the National Tuberculosis Molecular Surveillance Center located at the Michigan Department of Health.
For the next year, wgMLSType and GENType will be available to TB programs and uploaded into TB GIMS simultaneously. Cluster alerts will continue to be based on GENType for the next year to give TB programs time to acclimate and understand the changes. DTBE will make additional trainings available about TB GIMS and cluster alerts based on wgMLSType as the transition continues.
Please contact DTBESupport@cdc.gov with any questions or feedback.
Submitted by Steve Kammerer, MBA, BS, Statistician and Lauren Cowan, PhD, Senior Service Fellow
Jail Administrators Learn About the Importance of Collaboration with Public Health Officials to Prevent TB in Correctional Facilities
“Treatment of latent TB infection and the establishment of regular communications with local public health officials are key to the prevention of TB disease among people living in correctional facilities,” Lauren Lambert, MPH, BA told around 150 jail administrators at the American Jail Association’s 40th Annual Virtual Conference and Jail Expo, held virtually April 10–14, 2021.
TB disease disproportionately affects people who are incarcerated in the United States. They are 4–17 times more likely to have TB disease than the general population. The prevalence of latent TB infection can be as high as 25% among populations in correctional facilities. To highlight the importance of preventing TB disease in correctional facilities, Lauren was invited to present at the annual conference to share an overview of TB. Topics of the presentation included the risk of outbreaks associated with TB transmission in correctional and detention facilities, the importance of testing for latent TB infection in this population, and the availability of shorter treatment regimens for latent TB infection that increase the likelihood of treatment completion. Lauren also stressed the importance of planning as early as possible for persons being released from jail or prison, since, after release from correctional facilities, people who were formerly incarcerated face financial, housing, and employment needs that often take priority over health concerns. Finally, she encouraged the administrators to consider designating a TB liaison to communicate regularly with local public health officials and collaborate on TB control efforts for this population disproportionately affected by TB.
Submitted by Lauren Lambert, MPH, BA, Epidemiologist
2018 Contact Investigation Report
The 2018 Contact Investigation Report was recently distributed to TB stakeholders. The report summarizes national contact investigation efforts related to TB cases diagnosed in 2018, along with summary data from 2014-2017. The data is derived from the Aggregates Reports for Program Evaluation (ARPE) and Report of Verified Case of TB surveillance system. ARPE data provides the only national level data regarding TB prevention and control activities, such as contact investigation and treatment of latent TB infection.
National aggregate data from the 2018 ARPE report highlights contact investigation efforts in need of improvement. For example, the 2025 National TB Performance Target was not met for contact elicitation, contact examination, latent TB infection treatment initiation, or latent TB infection treatment completion. Examining the data further shows that two factors (contacts choosing to stop treatment and contacts lost to follow-up) accounted for most latent TB infection treatment completion failures each year 2014-2018.
Each TB program can evaluate their program’s contact investigation activities to (1) identify challenges to their contact investigation process, (2) design and implement strategies to overcome these challenges, (3) evaluate their progress and improve their contact investigation performance based on the National TB Program Objectives and Performance Targets.
Submitted by Tempest Hill, DrPH, MPH, Health Scientist

Dr. Beverly Metchock Named 2021 Association of Public Health Laboratories “On the Front Line” Award Winner
Congratulations to Dr. Beverly Metchock DrPH, D (ABMM), who was named the 2021 Association of Public Health Laboratories “On the Front Line” award winnerexternal icon! Dr. Metchock joined CDC in 1997 and has served as the Reference Laboratory Team Lead in DTBE’s Laboratory Branch since 2004. She received this nomination from state public health laboratory colleagues for her long-standing dedication to the TB community. Dr. Metchock is recognized for advancing the state of TB laboratory testing in the United States and globally as well as supporting U.S. public health laboratories through her consultative leadership.
Submitted by Angela M. Starks, PhD, Branch Chief
TB Elimination Alliance
The TB Elimination Alliance (TEA)external icon is a CDC-funded initiative to work more closely with state and local TB control programs as well as community-based organizations and community health centers. TEA’s mission is dedicated to eliminating TB and latent TB infection (LTBI) inequities among Asian, Asian American, Native Hawaiian, and Pacific Islander (A/AA & NH/PI) populations through education, raising awareness, and innovation. TEA’s goals are to conduct outreach to underserved A/AA & NH/PI communities with the highest TB burden, increase awareness and understanding of culturally and linguistically appropriate LTBI/TB testing and treatment strategies, share resources and best practices among providers, and develop partnerships to scale existing initiatives. TEA will be celebrating its one year anniversary in July 2021!
TEA recently expanded its membership to 15 organizations spanning across the continental United States, Hawai’i, and the U.S. Affiliated Pacific Islands. The newest member is from the Ministry of Health and Human Services, located in the Kwajalein Atoll of the Republic of the Marshall Islands. For more information about TEA’s members, visit https://tbeliminationalliance.org/external icon.
TEA is known for developing tailored and culturally responsive training programs to meet providers’ needs. Most recently, TEA hosted its first TB Learning Collaborative, an online training for an interdisciplinary cohort of clinical and non-clinical staff from community health centers, public health departments, and community-based organizations. The Learning Collaborative focused on quality improvement strategies to support standardized LTBI testing and treatment data collection efforts. The Learning Collaborative was guided by three TB subject matter experts: Dr. Amy Tang from North East Medical Services (CA), Dr. Fayette Nguyen Truax from Loma Linda University (CA), and Kara Green, MSN, APRN, FNP-BC from HOPE Clinic (TX). All cohort participants developed robust fishbone diagrams to identify root causes of stated problem areas, as well as S.M.A.R.T. goals to pursue meaningful solutions.
TEA is also known to offer a Mini-Grants Program to enhance LTBI and/or TB community engagement, provider education, and quality improvement activities for local organizations addressing TB in A/AA & NHPI communities. TEA will be wrapping up its first cycle of mini-grant awards this fall. Grant recipients have leveraged the funds to develop innovations and partnerships in TB education in hard-to-reach communities, TB screening strategies to identify high risk patients, and TB education and resources for new and current providers.
Visit https://tbeliminationalliance.org/events/category/webinars/list/?eventDisplay=pastexternal icon to learn more about the current mini-grant recipients (see March 9th and 11th webinar recordings). TEA announced a call for proposals for a second cycle of the Mini-Grants Program for the 2021-2022 fiscal year, and recipients will be announced this summer.
To learn more about TEA and inquire about collaboration opportunities, please contact tb-cen@aapcho.org or visit www.tbeliminationalliance.orgexternal icon.
Submitted by the Association of Asian Pacific Community Health Organizations — Evelyn Moua, Program Manager of TB Elimination and Joe Lee, MHSA, Director of Strategic Initiatives and Partnerships

Updated Resource: COVID-19 Case Investigation and Contact Tracing CDC’s Role and Approach
Case investigation and contact tracing are key strategies to stop the spread of COVID-19. Public health departments have used contact tracing for decades to slow or stop the spread of infectious diseases, such as TB, HIV, and sexually transmitted diseases (STDs).
CDC updated this 2-pager to provide recent information about COVID-19 vaccination and an updated list of CDC resources.
Learn more about CDC’s role and approach to case investigation and contact tracing for COVID-19.
DTBE-authored COVID-19 Publications
Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, Leung JW, Tsang CA, Pierce TJ, Kennedy JL, Hammett TA, Belay ED. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance studyexternal icon. Lancet Child Adolesc Health 20219:S2352–4642(21)00050–X. Epub ahead of print.
Demeke HB, Merali S, Marks S, Pao LZ, Romero L, Sandhu P, Clark H, Clara A, McDow KB, Tindall E, Campbell S, Bolton J, Le X, Skapik JL, Nwaise I, Rose MA, Strona FV, Nelson C, Siza C. Trends in use of telehealth among health centers during the COVID-19 pandemic—United States, June 26–November 6, 2020. MMWR Morb Mortal Wkly Rep 2021;70:240–4.
Fields VL, Kiphibane T, Eason JT, Hafoka SF, Lopez AS, Schwartz A, Henry A, Tran CH, Tate JE, Kirking HL, Laws RL, Venkatappa T, Mosites E, Montgomery MP. Assessment of contact tracing for COVID-19 among people experiencing homelessness, Salt Lake County health department, March–May 2020external icon. Ann Epidemiol 2021:S1047–2797. Epub ahead of print.
Godfred-Cato S, Tsang CA, Giovanni J, Abrams J, Oster ME, Lee EH, Lash MK, Le Marchand C, Liu CY, Newhouse CN, Richardson G, Murray MT, Lim S, Haupt TE, Hartley A, Sosa LE, Ngamsnga K, Garcia A, Datta D, Belay ED. Multisystem inflammatory syndrome in infants <12 months of age, United States, May 2020–January 2021external icon. Pediatr Infect Dis J 2021. Epub ahead of print
Goswami ND, Fiore AE, Walke HT. Evidence, experience, expertise, and the U.S. COVID-19 public health responseexternal icon. Clin Infect Dis 2021:ciab315. Epub ahead of print.
Hershow RB, Segaloff HE, Shockey AC, Florek KR, Murphy SK, DuBose W, Schaeffer TL Powell J, Gayle K, Lambert L, Schwitters A, Clarke KEN, Westergaard R. Rapid spread of SARS-CoV-2 in a state prison after introduction by newly transferred incarcerated persons — Wisconsin, August 14–October 22, 2020. MMWR Morb Mortal Wkly Rep 2021;70:478–482.
Hershow RB, Wu K, Lewis NM, Milne AT, Currie D, Smith AR, Lloyd S, Orleans B, Young EL, Freeman B, Schwartz N, Bryant B, Espinosa C, Nakazawa Y, Garza E, Almendares O, Abara WE, Ehlman DC, Waters K, Hill M, Risk I, Oakeson K, Tate JE, Kirking HL, Dunn A, Vallabhaneni S, Hersh AL, Chu VT. Low SARS-CoV-2 transmission in elementary schools—Salt Lake County, Utah, December 3, 2020–January 31, 2021. MMWR Morb Mortal Wkly Rep 2021;70:442–48.
Laws RL, Chancey RJ, Rabold EM, Chu VT, Lewis NM, Fajans M, Reses HE, Duca LM, Dawson P, Conners EE, Gharpure R, Yin S, Buono S, Pomeroy M, Yousaf AR, Owusu D, Wadhwa A, Pevzner E, Battey KA, Njuguna H, Fields VL, Salvatore P, O’Hegarty M, Vuong J, Gregory CJ, Banks M, Rispens J, Dietrich E, Marcenac P, Matanock A, Pray I, Westergaard R, Dasu T, Bhattacharyya S, Christiansen A, Page L, Dunn A, Atkinson-Dunn R, Christensen K, Kiphibane T, Willardson S, Fox G, Ye D, Nabity SA, Binder A, Freeman BD, Lester S, Mills L, Thornburg N, Hall AJ, Fry AM, Tate JE, Tran CH, Kirking HL. Symptoms and transmission of SARS-CoV-2 among children—Utah and Wisconsin, March–May 2020external icon. Pediatrics 2021;147:e2020027268.
Marks SM, Clara A, Fiebelkorn AP, Le X, Armstrong PA, Campbell S, Van Alstyne JM, Price S, Bolton J, Sandhu PK, Bombard JM, Strona FV. Influenza vaccination in health centers during the COVID-19 pandemic–United States, November 7–27, 2020external icon. Clin Infect Dis 2021:ciab318. Epub ahead of print.
McGovern OL, Stenger M, Oliver SE, Anderson TC, Isenhour C, Mauldin MR, Williams N, Griggs E, Bogere T, Edens C, Curns AT, Lively JY, Zhou Y, Xu S, Diaz MH, Waller JL, Clarke KR, Evans ME, Hesse EM, Morris SB, McClung RP, Cooley LA, Logan N, Boyd AT, Taylor AW, Bajema KL, Lindstrom S, Elkins CA, Jones C, Hall AJ, Graitcer S, Oster AM, Fry AM, Fischer M, Conklin L, Gokhale RH. Demographic, clinical, and epidemiologic characteristics of persons under investigation for Coronavirus Disease 2019—United States, January 17–February 29, 2020external icon. PLoS One 2021;16:e0249901.
Prince-Guerra JL, Almendares O, Nolen LD, Gunn JKL, Dale AP, Buono SA, Deutsch-Feldman M, Suppiah S, Hao LJ, Zeng Y, Stevens VA, Knipe K, Pompey J, Atherstone C, Bui DP, Powell T, Tamin A, Harcourt JL, Shewmaker PL, Medrzycki M, Wong P, Jain S, Tejada-Strop A, Rogers S, Emery B, Wang H, Petway M, Bohannon C, Folster JM, MacNeil A, Salerno R, Kuhnert-Tallman W, Tate JE, Thornburg NJ, Kirking HL, Sheiban K, Kudrna J, Cullen T, Komatsu KK, Villanueva JM, Rose, DA, Neatherlin JC, Anderson M, Rota PA, Honein MA, Bower WA. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites—Pima County, Arizona, November 3–17, 2020. MMWR Morb Mortal Wkly Rep 2021;70:100–5.
Self JL, Montgomery MP, Toews KA, Samuels EA, Imbert E, McMichael TM, Marx GE, Lohff C, Andrews T, Ghinai I, Mosites E; COVID-19 Homelessness Response Team. Shelter characteristics, infection prevention practices, and universal testing for SARS-CoV-2 at homeless shelters in 7 US urban areasexternal icon. Am J Public Health 2021:e1–6. Epub ahead of print.
Tompkins LK, Gunn JKL, Cherney B, Ham JE, Horth R, Rossetti R, Bower WA, Benson K, Hagan LM, Crist MB, Mettee Zarecki SL, Dixon MG, Dillaha JA, Patil N, Dusseau C, Ross T, Matthews HS, Garner K, Starks AM, Weiner Z, Bowen MD, Bankamp B, Newton AE, Logan N, Schuh AJ, Trimble S, Pfeiffer H, James AE, Tian N, Jacobs JR, Ruiz F, McDonald K, Thompson M, Cooley L, Honein MA, Rose DA; CDC COVID-19 Surge Diagnostic Testing Laboratory. Mass SARS-CoV-2 testing in a dormitory-style correctional facility in Arkansasexternal icon. Am J Public Health 2021:e1–10. Epub ahead of print.
Van Dyke ME, Mendoza MCB, Li W, Parker EM, Belay B, Davis EM, Quint JJ, Penman-Aguilar A, Clarke KEN. Racial and ethnic disparities in COVID-19 incidence by age, sex, and period among persons aged <25 years—16 U.S. jurisdictions, January 1–December 31, 2020. MMWR Morb Mortal Wkly Rep 2021;70:382–8.
Annan E, Stockbridge EL, Katz D, Mun EY, Miller TL. A cross-sectional study of latent tuberculosis infection, insurance coverage, and usual sources of health care among non–US-born persons in the United Statesexternal icon. Medicine (Baltimore) 2021;100:e24838.
Bryant KE, Yuan Y, Engle M, Kurbatova EV, Allen-Blige C, Batra K, Brown NE, Chiu KW, Davis H, Elskamp M, Fagley M, Fedrick P, Hedges KNC, Narunsky K, Nassali J, Phan M, Phan H, Purfield AE, Ricaldi JN, Robergeau-Hunt K, Whitworth WC, Sizemore EE; AIDS Clinical Trials Group; Tuberculosis Trials Consortium. Central monitoring in a randomized, open-label, controlled phase 3 clinical trial for a treatment-shortening regimen for pulmonary tuberculosisexternal icon. Contemp Clin Trials 2021:106355. Epub ahead of print.
Charles M, Richard M, Reichler MR, Koama JB, Morose W, Fitter DL. Treatment success for patients with tuberculosis receiving care in areas severely affected by Hurricane Matthew—Haiti, 2016external icon. PLoS One 2021;16:e0247750.
Deutsch-Feldman M, Pratt RH, Price SF, Tsang CA, Self JL. Tuberculosis—United States, 2020. MMWR Morb Mortal Wkly Rep 2021;70:409–14.
Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, Engle M, Goldberg SV, Phan HTT, Hakim J, Johnson JL, Lourens M, Martinson NA, Muzanyi G, Narunsky K, Nerette S, Nguyen NV, Pham TH, Pierre S, Purfield AE, Samaneka W, Savic RM, Sanne I, Scott NA, Shenje J, Sizemore E, Vernon A, Waja Z, Weiner M, Swindells S, Chaisson RE; AIDS Clinical Trials Group and the Tuberculosis Trials Consortium. Four-month rifapentine regimens with or without moxifloxacin for tuberculosisexternal icon. N Engl J Med 2021;384:1705–18
Feng PJ, Wu Y, Ho CS, Chinna L, Whelen AC, Largen A, Brostrom R, Reves R, Belknap R, Cattamanchi A, Banaei N. Impact of T-cell Xtend on T-SPOT.TB assay in high-risk individuals after delayed blood sample processingexternal icon. J Clin Microbiol 2021:JCM.00120–21. Epub ahead of print.
Harrist AV, McDaniel CJ, Wortham JM, Althomsons SP. Developing national genotype-independent indicators for recent Mycobacterium tuberculosis transmission using pediatric cases—United States, 2011–2017external icon. Public Health Rep 2021:33354920985215. Epub ahead of print.
Labuda SM, McDaniel C, Talwar A, Braumuller A, Parker S, McGaha S, Blissett C, Wortham J, Mukasa L, Stewart RJ. Tuberculosis outbreak associated with delayed diagnosis and long infectious periods in rural Arkansas, 2010–2018external icon. Public Health Rep 2021:33354921999167. Epub ahead of print.
Lewis NM, Salmanson AP, Price A, Risk I, Guymon C, Wisner M, Gardner K, Fukunaga R, Schwitters A, Lambert L, Baggett HC, Ewetola R, Dunn AC. Community-associated outbreak of COVID-19 in a correctional facility — Utah, September 2020–January 2021. MMWR Morb Mortal Wkly Rep 2021;70:467–472.
Mancuso JD, Miramontes R, Winston CA, Horsburgh CR Jr, Hill AN. Self-reported engagement in care among U.S. residents with latent tuberculosis infection—2011–2012external icon. Ann Am Thorac Soc 2021. Epub ahead of print.
Menzies NA, Shrestha S, Parriott A, Marks SM, Hill AN, Dowdy DW, Shete PB, Cohen T, Salomon JA. The health and economic benefits of tests that predict future progression to TB disease. Epidemiology. In press 2021.
Mirzazadeh A, Fellow IE, Ashki H, Parriott A, Readhead A, Barry P, Flood J, Navin TR, Hill AN, Reynolds S, Marks S, McCabe D, Mermin J, Kahn JG, Shete PB. Estimating Latent Tuberculosis Infection Prevalence in the United States: Back-Calculation from Active TB Cases. PLOS ONE. In press 2021. PMCID: PMC8016318.
Mirzazadeh A, Kahn JG, Haddad MB, Hill AN, Marks SM, Readhead A, Barry PM, Flood J, Mermin JH, Shete PB. State-level prevalence estimates of latent tuberculosis infection in the United States by medical risk factors, demographic characteristics and nativityexternal icon. PLoS One. 2021 Apr 1;16:e0249012.
Montgomery MP, Paulin HN, Morris A, Cotton A, Speers A, Boyd AT, Buff AM, Mathews D, Wells A, Marchman C, Gaffga N, Bamrah Morris S, Cavanaugh SS. Establishment of isolation and noncongregate hotels during COVID-19 and symptom evolution among people experiencing homelessness-Atlanta, Georgia, 2020external icon. J Public Health Manag Pract 2021;27:285–94.
Mosites E, Morris SB, Self J, Butler JC. Data sources that enumerate people experiencing homelessness in the United States: opportunities and challenges for epidemiological researchexternal icon. Am J Epidemiol 2021:kwab051. Epub ahead of print.
Mosites E, Morris SB, Self J, Butler J. Reply to comment on: data sources that enumerate people experiencing homelessness in the United States: opportunities and challenges for epidemiological researchexternal icon. Am J Epidemiol 2021:kwab105.
Narita M, Hatt G, Toren KG, Vuong K, Pecha M, Jereb JA, Goswami ND. Delayed tuberculosis diagnoses during the COVID-19 pandemic in 2020 — King County, Washingtonexternal icon. Clin Infect Dis 2021:ciab387. Epub ahead of print.
Parriott A, Kahn JG, Ashki H, Readhead A, Barry PM, Goodell AJ, Flood J, Shete PB. Modeling the Impact of Recommendations for Primary Care-Based Screening for Latent Tuberculosis Infection in Californiaexternal icon. Public Health Rep. 2020 Jul/Aug;135(1_suppl):172S-181S.
Rothenberg SE, Chen Q, Shen J, Nong Y, Nong H, Trinh EP, Biasini FJ, Liu J, Zeng X, Zou Y, Ouyang F, Korrick SA. Neurodevelopment correlates with gut microbiota in a cross-sectional analysis of children at 3 years of age in rural Chinaexternal icon. Sci Rep 2021;11:7384.
Scott NA, Lee KK, Sadowski C, Kurbatova EV, Goldberg SV, Nsubuga P, Kitshoff R, Whitelaw C, Thuy HN, Batra K, Allen-Blige C, Davis H, Kim J, Phan M, Fedrick P, Chiu KW, Heilig CM, Sizemore E; The AIDS Clinical Trials Group and The Tuberculosis Trials Consortium. Optimizing drug inventory management with a web-based information system: The TBTC Study 31/ACTG A5349 experienceexternal icon. Contemp Clin Trials 2021:106377. Epub ahead of print.
Self JL, McDaniel CJ, Bamrah Morris S, Silk BJ. Estimating and evaluating tuberculosis incidence rates among people experiencing homelessness, United States, 2007–2016external icon. Med Care 2021;59(Suppl 2):S175–81.
Williams W, Krueger A, Wang G, Patel D, Belcher L. The contribution of HIV testing funded by the Centers for Disease Control and Prevention to HIV diagnoses in the United States, 2010–2017external icon. J Community Health 2021. Epub ahead of print.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Meredith Moore at mue1@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.