TB NOTES

TB Notes 6, December 2018
Notes from the Director
Dear Colleague:

Looking back over 2018, I would like to thank the staff of CDC’s Division of Tuberculosis Elimination (DTBE) and our partners in public health for their collective efforts that led to another successful year in the fight to eliminate TB from the United States. A few highlights of our accomplishments and major activities of the year include the following:
- In recognition of World TB Day on March 24, DTBE recognized ten TB Elimination Champions who are leading efforts to collaborate with public health, health care providers, and organizations in their communities. DTBE also published preliminary TB surveillance data for the United States.
- The Tuberculosis Trials Consortium (TBTC) held their 41st Semi-Annual Meeting May 8-10 in Atlanta. The meeting highlighted research on the diagnosis, clinical management, and treatment of latent TB infection and TB disease.
- The ninth annual Tuberculosis Epidemiologic Studies Consortium (TBESC) meeting was held May 10-11 in Atlanta. The meeting highlighted current and planned TBESC studies that encompass elements of latent TB Infection testing, treatment, program evaluation, and opportunities to increase uptake of testing and treatment, such as working with community providers.
- In June, CDC released updated recommendations for the use of once-weekly isoniazid-rifapentine for 12 weeks (3HP) for the treatment of latent TB infection. The updated recommendations, published in CDC’s Morbidity and Mortality Weekly Report (MMWR), support expanded use of an effective, shorter, treatment regimen to reach even more people with latent TB infection.
- On September 26, 2018, the United Nations General Assembly held the first-ever High Level Meetingexternal icon focused on ending TB globally.
- The 2018 TB Education and Training Network (ETN) and the TB Program Evaluation Network (PEN) Conference, Breaking through Barriers to Achieve TB Elimination, was held September 18-20, in Decatur, Georgia.
- In October, DTBE released the 2017 surveillance data Reported Tuberculosis in the United States, 2017. We hope this report will be a useful tool in informing and improving TB prevention and control activities.
As we enter into next year, I continue to be inspired by your commitment to the very important work that you are doing every day. Thank you for everything you do in the fight to end TB. Happy New Year!
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division of Tuberculosis Elimination
2018 Year in Review
This year the Division of Tuberculosis Elimination (DTBE) would like to take a look back at the research, resources, and events that shaped an extraordinary year. Here is a look back at some TB highlights from 2018.

DTBE and state partners supported the emergency response to Hurricane Maria. On September 20, 2017, Hurricane Maria made landfall in Puerto Rico, as a Category 4 storm. The Puerto Rico Department of Health (PRDOH) facilities, including the PRDOH Institute of Laboratories in San Juan and the island’s health system, suffered significant damage. With assistance from the DTBE Laboratory Branch and Field Services Branch; CDC’s Emergency Operations Center; CDC Foundation; and Association of Public Health Laboratories, a shipping logistics plan was developed. TB specimens were shipped from Puerto Rico to CDC, a triage site for shipment of specimens to Georgia, Florida, and Virginia public health laboratories, to support diagnostic and confirmatory testing between October 2017 and early February 2018.

On March 24, we celebrated World TB Day (WTBD). The U.S. theme for World TB Day 2018 was “Wanted: Leaders for a TB Free United States. We can make history. End TB.” The Division of Tuberculosis Elimination (DTBE) World TB Day activities highlighted our achievements, present efforts, and future aspirations. Activities included TB Chronicles project, CDC Instagram Takeover, WTBD Thunderclap, and the U.S TB Elimination Champions Project. Our website featured over 40 World TB Day activities submitted to DTBE taking place in 15 states.

TBTC was a runner up for the Kochon Prize 2017 for their work on TB drugs. The Kochon Prize has been given annually for the past 11 years to individuals and/or organizations that have made a highly significant contribution to ending TB.

DTBE released updated recommendations for the use of once-weekly isoniazid-rifapentine for 12 weeks (3HP) for the treatment of latent tuberculosis (TB) infection. The updated recommendations, published in CDC’s Morbidity and Mortality Weekly Report (MMWR), support expanded use of an effective, shorter, treatment regimen to reach even more people with latent TB infection.
The updated recommendations include the use of 3HP:
- By directly observed therapy or self-administered therapy in persons over 2 years of age,
- In children and adolescents, 2-17 years old, and
- In persons with latent TB infection and HIV/AIDS and taking antiretroviral medications with acceptable drug interactions with rifapentine.

DTBE established a TB Emergency Drug Stockpile (TEDS) that has already been put to the test. Although the stockpile was created to help TB programs weather manufacturer drug shortages, recently, release of drugs from the stockpile was needed in two other situations. The first involved providing drugs from the stockpile that were soon expiring to TB programs so that they could be used in treatment before they expired. The second involved providing rifapentine (RPT) to TB programs experiencing interruption in supplies from their distributor.

The 2018 TB Education and Training Network (ETN) and the TB Program Evaluation Network (PEN) Conference, Breaking through Barriers to Achieve TB Elimination, was held September 18-20, 2018, in Decatur, Georgia. This year’s conference highlighted common aspects of TB education, training, and evaluation.

On September 26, 2018, the United Nations General Assembly held the first-ever High Level Meeting (UN HLM)external icon focused on ending TB globally. CDC leadership and global leaders met to discuss the need for increased efforts to test and treat persons with latent TB infection to prevent TB disease. CDC continues to be at the forefront of researching new diagnostics and treatment for TB, with our global efforts informed and strengthened by our domestic accomplishments.

DTBE released the 2017 surveillance report, entitled Reported Tuberculosis in the United States, 2017. A total of 9,105 TB cases were reported in the United States in 2017. Although the 2017 case count represents the lowest number of TB cases on record in the United States, too many people still suffer from TB disease.

The Division of Tuberculosis Elimination (DTBE) staff, in collaboration with colleagues from the New York City Bureau of Tuberculosis Control, presented preliminary findings from the eDOT study at the 49th Union World Conference on Lung Health – The Hague, The Netherlands. During the conference’s Rapporteur Session, the TB Section highlighted the eDOT study to raise conference attendees’ awareness of these early findings.
Submitted by Joan Mangan, PhD, DTBE
Virginia’s Annual TB and Refugee Nurse Training, Focusing Below the Surface

The Virginia Department of Health’s Division of TB and Newcomer Health (DTBNH) hosted their annual TB and Refugee Nurse Training September 25-27 with the theme of TB Prevention: Focusing Below the Surface. Around 80 participants from the 35 Virginia health districts attended with many others viewing online. In person, attendees had opportunities to meet, share experiences and network with central office staff, guest speakers and their peers in other parts of the state.
Speakers from the CDC, Virginia State Lab, University of Virginia, local Virginia health departments, Department of Social Services, Global TB Institute, and Resettlement agencies presented on a myriad of topics such as: laboratory technology, case management, community engagement, LTBI treatment updates and educational messaging to improve acceptance and adherence, contact investigations, working with Afghans, and the US refugee admissions process. The evening of the 25th, the group enjoyed a screening of the documentary, Talking Story, which chronicled the lives, rituals and wisdom of traditional healers and spiritual leaders from diverse world cultures.

Spittoon Award Recipients
To wrap up the event, awards were given to Health Districts for both Tuberculosis and Newcomer Health categories. The awards presented were: the Newcomer Health Excellence award – awarded based on nominations from local resettlement agencies, the “B” award – awarded based on performance for evaluating TB classified individuals, and the Spittoon award – awarded based on performance on NTIP indicators.

B Award Recipients
Participants completed evaluations of the training and they have been encouraging. The feedback suggest the DTBNH continue to provide this type of intense annual training, giving feedback such as, “The clinical presentations by faculty were outstanding. I appreciated the expertise of external partners,” “The best ever!” and “Very good – a lot of work went into this and it is appreciated! Thanks for all that you do for us and for your responsiveness to our questions.”

Newcomer Health Excellence Award
Submitted by Amanda Khalil and Denise Dodge, Virginia Department of Health
2017 Surveillance Report Overview
TB remains under control in the United States, but the rate of decline in the United States remains too slow to achieve TB elimination in this century.

Key Findings
A total of 9,105 TB cases were reported in the United States in 2017 according to data from the CDC National TB Surveillance System. This represents a decrease of 148 from the 9,253 cases reported in 2016. TB cases were reported in all fifty states and the District of Columbia. The 2017 case count represents the lowest number of TB cases on record in the United States. The national incidence rate was 2.8 cases per 100,000 persons (1.6% decrease from 2016).
TB in Specific Populations
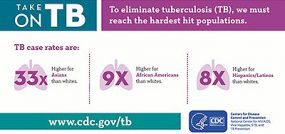
TB disease remains more common among people who were born in countries with high rates of TB. The percentage of TB cases among non-U.S.–born persons who have been in the United States for 10 years or longer are about equal to those who have been in the United States less than 10 years. Minority populations continue to disproportionately bear the burden of TB disease.
TB Treatment
Prompt and effective treatment of TB disease is critical to prevent ongoing transmission of TB. A typical case of drug-susceptible TB in the United States costs $19,000 to treat and requires at least 180 days of medication, plus x-rays, lab tests, and follow-up and testing of contacts.
Drug-Resistant TB
Drug-resistant TB is complex and costly to treat. A single case of multidrug-resistant TB costs $164,000 to treat. Treatment costs for one case of extensively drug-resistant TB are $526,000. In 2017, there were 121 cases of multidrug-resistant TB and 2 cases of extensively drug-resistant TB reported in the United States. The percentage of TB cases that are drug resistant has remained stable for the last 20 years.
Surveillance Report Resources
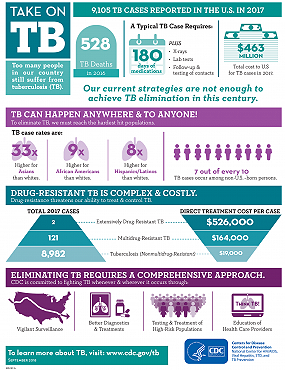
CDC has developed communication resources with highlights from the 2017 Surveillance Report for state and local TB programs. We encourage state and local TB programs and partners to use these materials to talk about TB in your communities, and to continue the important work towards TB elimination.
Tuberculosis Whole Genome Sequencing Training Modules

In 2018, the Division of Tuberculosis Elimination (DTBER) began universal whole-genome sequencing (WGS) (i.e., sequencing an M. tuberculosis isolate for each U.S. case of culture-confirmed TB). Increasingly, state and local TB programs are using the results of WGS and phylogenetic analyses to help guide TB cluster and outbreak investigations.
DTBE created Tuberculosis Whole Genome Sequencing Modules to provide state and local TB program staff with information on the current uses and plans for WGS for the investigation of recent TB transmission in the U. S.
- Part 1. Using WGS for detection and investigation of recent TB transmissionexternal icon
- Part 2. Case studies using WGS to investigate TB cluster alerts in Californiaexternal icon
- Part 3. Plans for transition to universal prospective WGSexternal icon
Submitted by Benjamin Silk, PhD, DTBE
Systems Thinking Symposium at the University of North Texas Health Science Center
DTBE representatives came together with healthcare professionals and others from across the United States for a Systems Thinking Symposiumexternal icon at the University of North Texas Health Science Center (UNTHSC). The goal of the symposium was to engage participants in multifaceted thinking, to look at new ways of addressing latent TB infection. The symposium included participants from across Texas and other states bringing perspectives on latent TB testing and treatment, representing academia and research, healthcare, state and federal health agencies, private and public provider organizations, the managed care industry, and others. A discussion on latent TB infection noted the importance of collaborations, “The public health system does not have the capacity to address this alone,” said Dr. Thomas R. Navin, Chief of the DTBE Surveillance, Epidemiology, and Outbreak Investigations Branch, who opened the symposium at UNTHSC.
Submitted by Sloane Bowman, MPH, DTBE
New Microbiologists in the Laboratory Branch

The laboratory branch is excited to announce the addition of four new staff members.
On August 6, 2018, Scott Chancey, Ph.D. joined the Reference Laboratory Team (RLT). Scott will perform molecular testing for the Molecular Detection of Drug Resistance (MDDR) service. Scott has worked as a molecular biologist on the Aseptic Meningoencephalitis Project in the Division of Preparedness & Emerging Infections, Laboratory Preparedness & Response Branch. He also spent 10 years as a Senior Research Associate and Pneumococcal Genetics and Epidemiology Team Lead at Emory University School of Medicine. Scott earned his B.S. and M.S. in Microbiology from Auburn University and his Ph.D. in Plant Biology and Genetics from the University of Arizona.
On September 4, 2018, Robert Domaoal, Ph.D. joined the Laboratory Capacity Team (LCT). As a Laboratory Consultant, Rob will provide oversight and technical consultation to 14 public health TB laboratories funded through the DTBE cooperative agreement. Rob spent two years in CDC’s Center for Global Health in the Division of Global HIV and TB, International Laboratory Branch. He also worked at Emory University in HIV research. Rob earned his B.A. from Boston University and his M.S. and Ph.D. from the University of Rochester.
On September 17, 2018, Wen Li, Ph.D. joined the Applied Research Team (ART). Wen will perform immunology research activities for the development of new methods to screen novel host-directed and pathogen-directed therapeutics against tuberculosis. Wen spent 4 years specializing in transcriptomics and genomics at CDC and 7 years as a Graduate Research Assistant at Georgia State University’s Biochemistry Laboratory where she worked on bacterial multidrug resistance. Wen earned her B.S. in Biology from China Agricultural University and her Ph.D. in Molecular Genetics and Biochemistry from Georgia State University.
On October 1, 2018, Richard Ebai, joined RLT. Richard will perform growth-based drug susceptibility testing for the Molecular Detection of Drug Resistance (MDDR) service. Richard spent 10 years as a Microbiology Medical Technologist in hospital systems. Richard earned his B.S. in Zoology from Dschang University in Cameroon. He also earned his Certificate in Medical Technology from Shands Jacksonville School of Medical Technology and his M.S in Clinical Laboratory Science from University of Southern Mississippi.
Please welcome Scott, Rob, Wen, and Richard to DTBE.
Submitted by Tracy Dalton, PhD, DTBE
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Sloane Bowman at wnv2@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates here.
Cherng ST, Shrestha S, Reynolds S, Hill AN, Marks SM, Kelly J, Dowdy DW. Tuberculosis incidence among populations at high risk in California, Florida, New York, and Texas, 2011–2015.external icon Am J Public Health 2018; 108(Suppl 4):S311–4.
Chorba T. Trench conflict with combatants and infectious disease [Note]. Emerg Infect Dis 2018;24:2136–7.
Ezewudo M, Borens A, Chiner-Oms Á, Miotto P, Chindelevitch L, Starks AM, Hanna D, Liwski R, Zignol M, Gilpin C, Niemann S, Kohl TA, Warren RM, Crook D, Gagneux S, Hoffner S, Rodrigues C, Comas I, Engelthaler DM, Alland D, Rigouts L, Lange C, Dheda K, Hasan R, McNerney R, Cirillo DM, Schito M, Rodwell TC, Posey J. Integrating standardized whole genome sequence analysis with a global Mycobacterium tuberculosis antibiotic resistance knowledgebase.external icon Sci Rep 2018;8:15382.
Haddad MB, Raz KM, Lash TL, Hill AN, Kammerer JS, Winston CA, Castro KG, Gandhi NR, Navin TR. Simple estimates for local prevalence of latent tuberculosis infection, United States, 2011–2015. Emerg Infect Dis 2018;24:1930–3.
Iqbal SA, Winston CA, Bardenheier BH, Armstrong LR, Navin TR. Age-period-cohort analyses of tuberculosis incidence rates by nativity, United States, 1996–2016. Am J Public Health 2018external icon;108(Suppl 4):S315–20.
Khan A, Marks S, Katz D, Morris SB, Lambert L, Magee E, Bowman S, Grant G. Changes in tuberculosis disparities at a time of decreasing tuberculosis incidence in the United States, 1994–2016. Am J Public Health 2018;108(Suppl 4):S321–6.
Lowenthal P, Lin SG, Desmond E, Shah N, Flood J, Barry PM. Evaluation of the impact of a sequencing assay for detection of drug resistance on the clinical management of tuberculosis.external icon Clin Infect Dis 2018. Epub ahead of print.
Macaraig, M.M., Jalees, M., Lam, C., and Burzynski, J. Improved treatment completion with shorter treatment regimens for latent tuberculous infection.external icon 02838. 2018 Int J Tuberc Lung Dis (22):11
The CRyPTIC Consortium and the 100,000 Genomes Project. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing.external icon N Engl J Med 2018;379:1403–15.