TB NOTES

TB Notes 4, 2020
Notes from the Director

Dear Colleague,
The end of the year marks a time to reflect on achievements, and an opportunity to look forward to future goals. The year 2020 was a year no one could have predicted. Throughout 2020, the COVID-19 pandemic impacted public health services, including tuberculosis (TB) control services. The COVID-19 pandemic caused a shift in staff roles and responsibilities as well as resources in public health departments and healthcare facilities across the United States to address the ongoing needs of the COVID-19 response.
As of December 14, 110 DTBE staff members (69% of DTBE staff) have participated in 221 deployments supporting CDC’s COVID-19 response efforts. Over 650 staff from the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention have been deployed to work on COVID-19 response activities. DTBE’s embedded field-based staff have also participated in the response efforts of their assigned local jurisdictions. Thank you to everyone who has responded to the COVID-19 pandemic.
On December 8-9, 2020, the Advisory Council for the Elimination of Tuberculosis (ACET) convened to provide advice and recommendations regarding the elimination of TB in the United States. ACET received updates and information from the Division of Tuberculosis Elimination and discussed various TB-related topics.
In 2020, I saw many outstanding efforts in the service of our collective mission to prevent, control, and eventually eliminate TB in the United States. TB staff have risen to the challenges of 2020. Thank you for your continued commitment and all that you have done this year.
I am proud to work alongside you as we apply innovation and scientific excellence to fulfill our mission and implement strategies for TB elimination. Best wishes for a happy and healthy new year!
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
This year the Division of Tuberculosis Elimination (DTBE) would like to reflect on the research, resources, resilience, and world events that shaped a momentous year. Here is a look back at some TB highlights for 2020.
In February 2020, CDC and the National Tuberculosis Controllers Association (NTCA) published “Guidelines for the Treatment of Latent Tuberculosis Infection” in CDC’s Morbidity and Mortality Weekly Report Recommendations and Reports. This was the first comprehensive update to U.S. latent TB infection treatment guidelines since 2000. CDC and NTCA preferentially recommend short-course, rifamycin-based, 3- or 4-month latent TB infection treatment regimens over 6- or 9-month isoniazid monotherapy.

On March 24, DTBE recognized World TB Day, an annual event to commemorate the date in 1882 when Dr. Robert Koch announced his discovery of Mycobacterium tuberculosis, the bacillus that causes tuberculosis (TB). The 2020 World TB Day theme was “It’s Time!” Activities in 2020 included a Dear Colleague Letter from CDC Director, Dr. Robert Redfield, M.D., a new World TB Day video of five TB survivors and family of survivors bravely sharing their experiences battling latent TB infection and TB disease, the CDC U.S. TB Elimination Champions Project, a World TB Day Social Media Photo Challenge, and more.
In April, CDC posted an updated summary of recommendations for latent TB infection testing and treatment in the United Statespdf icon. The updates describe who should be tested, the types of recommended tests for TB infection and the recommended treatment regimens for latent TB infection. Medscape published a “5 Things to Know” article, “Latent TB Infection: New Guidelines and Preferred Treatmentsexternal icon,” that describes five key points from the new latent TB infection treatment guidelines. Also, MMWR and Medscape partnered to release free continuing education (CE) credit for the updated latent TB infection treatment guidelinesexternal icon.
In May, for the first time in the history of the Tuberculosis Trials Consortium (TBTC)external icon, DTBE’s Clinical Research Branch (CRB) held the TBTC 45th Semi-Annual Meeting as a virtual meeting. The meeting was held on the Zoom platform and was a great success with approximately 150 participants worldwide.
In October, results from two important studies were presented at the 51st Union World Conference:
- CDC’s Tuberculosis Trials Consortium (TBTC) and the National Institute of Health’s Adult AIDS Clinical Trials Group (ACTG) announced findings from Study 31/A5349. Study 31/A5349 is the first clinical trial to identify a shorter 4-month daily treatment regimen for drug-susceptible TB disease that is as effective as (non-inferior to) the existing 6-month daily regimen in curing TB disease. This is the first new treatment regimen for drug-susceptible TB disease in almost 40 years. Read the Dear Colleague Letter for more information.
- A two-year study conducted by New York City Department of Health and Mental Hygiene, CDC, and Columbia University, showed that electronic directly observed therapy (eDOT) was as effective as traditional in-person DOT for ensuring high adherence to treatment while enabling patient-centered care for TB disease.
Also in October, CDC released the 2019 edition of Reported Tuberculosis in the United States. Once again, the United States reported the lowest number of TB cases (8,916) and the lowest incidence rate on record (2.7 cases per 100,000 persons). The United States has achieved substantial progress in reducing TB; however, the rate of decrease remains low. This report compiles TB surveillance data for U.S. TB cases counted in 2019, before the COVID-19 pandemic directly affected the United States.
In November, CDC joined the TB Community Engagement Network for the first TB Summit. The virtual summit brought together partners from community health centers, community-based organizations, academic partners, state and local TB programs, and others to share best practices and lessons learned.
Throughout the year, DTBE staff members responded to the COVID-19 pandemic as did many TB program staff. As of December 14, 2020, 110 DTBE staff members (69% of DTBE staff) have participated in 221 deployments supporting CDC’s COVID-19 response efforts. Over 650 staff from the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention have been deployed to work on COVID-19 response activities. DTBE’s embedded field-based staff have also participated in the response efforts of their assigned local jurisdictions. As contract tracing approaches from other disease areas, such as TB, were adapted and integrated into the COVID-19 response, DTBE and the TB Centers of Excellence provided resources including training courses, guides, and patient education materials for TB programs that could be adapted for strategies in responding to the COVID-19 pandemic.
Updated Mantoux tuberculin skin test (TST) factsheet available

DTBE has released the updated Tuberculin Skin Testing factsheetpdf icon. The factsheet has a new look that is easy to read. The online version is a digital-first factsheet with dropdown options to frequently asked questions.
Submitted by Jasmine Kenney, MPH
TB ETN Webinar
The TB Education and Training Network webinar, Effectively Converting in-Person Training to a Virtual Format, was held on December 17th. Stefani Nixon, a TB Education and Training Specialist from the Southeastern National TB Center, presented a step-by-step process designed to effectively convert in-person training to a virtual format. Stefani’s presentation was followed by Tavia Mirassou-Wolf, a TB Training and Education Coordinator from the Colorado Department of Public Health and Environment, who presented a case study demonstrating how the Colorado TB Program delivered a virtual TB 101 course. The webinar concluded with a question and answer session led by Ann Scarpita, a TB Nurse Consultant from the Colorado Department of Public Health and Environment. The webinar was attended by health educators who design TB education and training. A recording will be available on the NPIN website.
Submitted by Peri Hopkins, MPH
Strategies toward Meeting National Objectives for Completion of Treatment for Contacts to Sputum AFB Smear-Positive Tuberculosis Cases
Each year the Kentucky Department for Public Health’s Tuberculosis Prevention and Control Program (KTP) evaluates progress towards achieving the national TB objective: “For contacts to sputum AFB smear-positive TB cases, who have started treatment for latent tuberculosis infection (LTBI), increase the proportion who complete treatment to 81% by 2020.”
In 2015, the KTP identified that only 54% of contacts completed LTBI therapy. KTP conducted an evaluation specifically focused on the impact of activities currently being used by local staff to promote completion of therapy. We found that frequent staffing turnover resulted in patients who moved outside of their jurisdiction often being “lost to follow-up.” New staff did not realize the need to timely coordinate care so that staff in new jurisdictions could actively engage contacts.
Upon reflection on our evaluation efforts, we identified the following facilitators and lessons learned for successful program evaluation:
- Establish small realistic yearly state benchmarks for each objective, to motivate staff and show steady achievement
- Enhance biannual trainings for newly hired local staff, to stress the importance of timely submission of interjurisdictional notifications to assure coordination of care for patients who relocate
- Promote use of state and local incentives and enablers by highlighting information in biannual newsletters and disseminating request forms to all local clinics
- Revise data collection tools for local staff to use, to assure data is accurate; and assess if annual evaluation activities are valid, realistic, and practical
In 2018, the KTP dedicated its efforts to expanding surveillance expertise by engaging a full-time epidemiologist who could focus time and effort on the evaluation of barriers towards contacts completing therapy. Kentucky has been classified as having a medium-incidence for reporting active tuberculosis cases; therefore gaining a dedicated epidemiologist would enhance current surveillance activities.
As a result of each of these efforts, Kentucky showed remarkable improvement towards meeting its own benchmarks (2016 71%, and 2017 74 %) for completion of treatment among contacts to sputum smear-positive TB patients. Finally, in 2018, Kentucky met the 2020 national indicator with an 81% completion rate. Preliminary 2019 data predict that Kentucky will once again meet the 2020 national goal. Ongoing evaluation strategies and activities will assure that timely assessment, use of incentive and enablers to engage patients, and enhancing nurse-patient relationships will promote completion of therapy and effectively assure prevention of active TB disease.
Submitted by:
KY Tuberculosis Prevention and Control Program
Emily Anderson RN, BSN; Charles H. Rhea MPH; and Maria Lasley RN, BSN, MA, MBA
Improving TB Prevention and Control through Program Evaluation: New Web Tools Available for TB Programs
The Program Evaluation and Health Economics Team of the Division of Tuberculosis Elimination (DTBE) is delighted to introduce new program evaluation tools for TB Programs. These tools are available on the CDC website at https://www.cdc.gov/tb/programs/evaluation/default.htm. The website features tools that are TB-specific, information to support evaluation activities, and links to resources for TB incidence modeling and estimation of costs. The tools are aligned with the 2025 National TB Program Objectives and Performance Targets. The tools can be used to identify an evaluation focus area using the National Tuberculosis Indicators Project (NTIP), to develop an evaluation plan, and to form evaluation objectives and evaluation questions. The website also provides information about TB data sources and frequently asked questions about program evaluation. The Program Evaluation and Health Economics team is continually developing tools to support the program evaluation needs of TB Programs. Tools expected to be added to the website soon include guides to creating a logic model for evaluation, using evaluation findings, using modeling to assist with creating TB elimination plan, and calculating costs of Video Directly Observed Therapy. Additional information will also be added about data submission of the Aggregate Reports for Tuberculosis Program Evaluation (ARPE) forms on contact investigation and targeted testing.
Submitted by Eva Trinh, PhD, MPH Health Scientist
Model Performance Evaluation Program (MPEP)
As part of the continuing effort to assess and monitor the quality and effectiveness of laboratory testing systems that support public health objectives of tuberculosis (TB) treatment programs, the CDC Model Performance Evaluation Program (MPEP) was established to analyze the performance and practices of all known clinical and public health laboratories in the United States that perform drug susceptibility testing of isolates belonging to the Mycobacterium tuberculosis complex (MTBC). MPEP is an educational self-assessment tool in which five isolates of M. tuberculosis complex (MTBC) are sent to participating laboratories biannually for staff to monitor their ability to detect drug resistance among the isolates. It is not a formal, graded proficiency testing program; however, results are used by laboratories for their quality assurance programs and staff competencies.
DTBE recently updated the MPEP website for a user-friendly, easy to read experience. Visit the updated MPEP website to learn more about the program and see the latest program results.
Submitted by Monica Youngblood, Microbiologist
TB Community Engagement Network Announces Mini-Grant Program Awardees
The Tuberculosis Community Engagement Network (TB CEN) is excited to announce this year’s Mini-Grants Program Awardees! The TB CEN offers mini-grants to organizations serving Asian American (AA), Native Hawaiian and Pacific Islander (NHPI) communities to increase awareness and build provider capacity on latent tuberculosis infection (LTBI) and tuberculosis (TB) testing and treatment. This year’s Mini-Grants program prioritizes activities that enhance LTBI and/or TB community engagement and education, provider education, and quality improvement.
The Mini-Grants Program awardees represent 10 organizations located across AA and NHPI communities with the highest TB burden. The awarded organizations are as follows:
- Arkansas Coalition of Marshallese (Springdale, AR)
- Asian American Community Services (Columbus, OH)
- Asian American Health Coalition of the Greater Houston Area, dba HOPE Clinic (Houston)
- Asian Services In Action, Inc. (Akron, OH)
- Center for Pan Asian Community Services, Inc. (Atlanta)
- Colorado Department of Public Health and Environment (Denver)
- Fort Bend County Clinical Health Services (Rosenberg, TX)
- North East Medical Services (Daly City, CA)
- San Diego County Medical Society Foundation, dba Champions for Health (San Diego, CA)
- UCSF Benioff Children’s Hospital Oakland (Oakland, CA)
Congratulations to the TB CEN Mini-Grants Program Awardees!
To learn more about the TB CEN and the Mini-Grants Program, visit the Association of Asian Pacific Community Health Organizations (AAPCHO) website –https://aapcho.org/national-network-aims-to-eliminate-tuberculosis-across-at-risk-communities/external icon
First annual virtual 2020 TB Community Engagement Network Summit held November 18-19, 2020
The first annual Tuberculosis (TB) Community Engagement Network (CEN) Summit was held virtually November 18-19, 2020. The theme of this year’s Summit was “Eliminating TB Together” and focused on outreach to Asian American, Native Hawaiian, and Pacific Islander communities at the highest risk of latent TB infection and TB disease.
Learn more: https://aapcho.org/national-network-aims-to-eliminate-tuberculosis-across-at-risk-communities/external icon
Submitted by Leeanna Allen, MPH
Prioritizing Case Investigations and Contact Tracing for COVID-19 in High Burden Jurisdictions
On November 23, 2020, CDC released new recommendations prioritizing case investigations and contact tracing to assist health departments experiencing escalating COVID-19 cases. These new recommendations further refine existing prioritization recommendations and presents new modeling data regarding timeframes to maximize the effectiveness of contact tracing activities.
The new case investigation and contact tracing prioritization recommendations are available on CDC’s web site at: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/prioritization.html
Key prioritization recommendations include:
- Prioritize case investigation interviews for people diagnosed with COVID-19 in the past 6 days (based on specimen collection date or symptom onset, if known);
- Prioritize contact tracing efforts on household contacts exposed in past 6 days, and people living, working or visiting congregate living facilities, high density workplaces or other settings (or events) with potential extensive transmission;
- If more than 14 days have elapsed since the specimen was collected, case investigation should generally not be pursued.
Please see a summary of recommendations for prioritization of COVID-19 case investigation and contact tracing in the figure below.
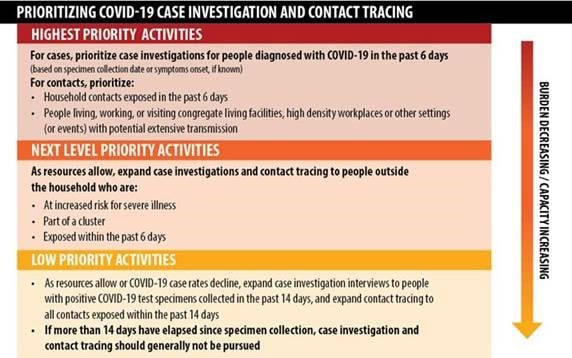
Case investigation and contract tracing remain essential components of the COVID-19 response and are a key strategy to interrupt disease transmission and reduce spread of the virus in a community. As the burden of COVID-19 worsens in an area, and the capacity to investigate new cases in a timely manner becomes more difficult or infeasible, health departments should prioritize which cases to investigate and contacts to trace, and emphasize broader community mitigation measures. Implementation should be guided by what is feasible, practical, and acceptable, as well as tailored to the needs of each community.
For more information and resources on Contact Tracing, refer to Contract Tracing Resources for Health Departments at: https://www.cdc.gov/coronavirus/2019-ncov/php/open-america/contact-tracing-resources.html
The Contact Tracing Program Support Team is available to provide technical assistance, and can be reached at CDC IMS NCOV Response STLT Clearance eocevent423@cdc.gov

New CDC Contact Tracing Video
CDC is pleased to announce the release of a new video titled “Answer the Call” describing the contact tracing process. This video can be found on CDC’s Contact Tracing Resources for Health Departments webpage as well as in the Contact Tracing Communications Toolkit for Health Departments.
A Spanish translation of the video was released December 10, 2020.
This 1-minute animated video informs the public about contact tracing and why they should answer and respond to a call from a public health worker. The video:
- Explains what will happen when a person with COVID-19 receives a call from a public health worker to initiate contact tracing
- Highlights that a public health worker will check on the person’s health, answer questions, and explain how a public worker will ask about where the person with COVID-19 has been and who they have been around to help identify close contacts
- Addresses potential concerns that may make some people reluctant to participate in contact tracing, addresses misinformation around contact tracing, and explains that all information provided by the participant during the call is confidential and kept private

This new video is an addition to existing contact tracing communication resources that includes
- general information
- questions and answers on contact tracing
- infographics
- social media resources and graphics.
DTBE-authored COVID-19 Publications
Bamrah MS, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, Lee EH, Paneth-Pollak R, Geevarughese A, Lash MK, Dorsinville MS, Ballen V, Eiras DP, Newton-Cheh C, Smith E, Robinson S, Stogsdill P, Lim S, Fox SE, Richardson G, Hand J, Oliver NT, Kofman A, Bryant B, Ende Z, Datta D, Belay E, Godfred-Cato S. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1450–6. doi:10.15585/mmwr.mm6940e1external icon
Chorba T. Social distancing and artful pandemic survivalexternal icon. Emerg Infect Dis. 2020;26:2793–4. doi: 10.3201/eid2611.ac2611
Chorba T. The concept of the crown and its potential role in the downfall of coronavirus. Emerg Infect Dis. 2020; 26:2302–5. doi: 10.3201/eid2609.ac2609
Delahoy MJ, Whitaker M, O’Halloran A, Chai SJ, Kirley PD, Alden N, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Monroe ML, Ryan PA, Fox K, Kim S, Lynfield R, Siebman S, Davis SS, Sosin DM, Barney G, Muse A, Bennett NM, Felsen CB, Billing LM, Shiltz J, Sutton M, West N, Schaffner W, Talbot HK, George A, Spencer M, Ellington S, Galang RR, Gilboa SM, Tong VT, Piasecki A, Brammer L, Fry AM, Hall AJ, Wortham JM, Kim L, Garg S, COVID-NET Surveillance Team. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19—COVID-NET, 13 states, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1347–54. doi: 10.15585/MMWR.MM6938E1external icon
Hughes MM, Groenewold MR, Lessem SE, Xu K, Ussery EN, Wiegand RE, Qin X, Do T, Thomas D, Tsai S, Davidson A, Latash J, Eckel S, Collins J, Ojo M, McHugh L, Li W, Chen J, Chan J, Wortham JM, Reagan-Steiner S, Lee JT, Reddy SC, Kuhar DT, Burrer SL, Stuckey MJ. Update: characteristics of health care personnel with COVID-19—United States, February 12–July 16, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1364–8. doi: 10.15585/MMWR.MM6938A3
Kambhampati AK, O’Halloran AC, Whitaker M, Magill SS, Chea N, Chai SJ, Kirley PD, Herlihy RK, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Monroe ML, Ryan PA, Kim S, Reeg L, Como-Sabetti K, Danila R, Davis SS, Torres S, Barney G, Spina NL, Bennett NM, Felsen CB, Billing LM, Shiltz J, Sutton M, West N, Schaffner W, Talbot HK, Chatelain R, Hill M, Brammer L, Fry AM, Hall AJ, Wortham JM, Garg S, Kim L; COVID-NET Surveillance Team. COVID-19–associated hospitalizations among health care personnel—COVID-NET, 13 states, March 1–May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1576–83. doi: 10.15585/mmwr.mm6943e3external icon
Koonin LM, Hoots B, Tsang CA, Leroy Zanie, Farris K, Jolly B, Antall P, McCabe B, Zelis CBR, Tong I, Harris AM. Trends in the use of telehealth during the emergence of the COVID-19 pandemic—United States, January–March 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1595–9. doi: 10.15585/mmwr.mm6943a3external icon
Leeb RT, Price S, Sliwa S, Kimball A, Szucs L, Caruso E, Godfred-Cato S, Lozier M. COVID-19 trends among school-aged children—United States, March 1–September 19, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1410–5. doi: 10.15585/mmwr.mm6939e2external icon
Schwartz NG, Moorman AC, Makaretz A, Chang KT, Chu VT, Szablewski CM, Yousaf AR, Brown MM, Clyne A, DellaGrotta A, Drobeniuc J, Korpics J, Muir A, Drenzek C, Bandy U, Kirking HL, Tate JE, Hall AJ, Lanzieri TM, Stewart RJ. Adolescent with COVID-19 as the source of an outbreak at a 3-week family gathering—four states, June–July 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1457–9. doi: 10.15585/mmwr.mm6940e2external icon
Wilson RF, Sharma AJ, Schluechtermann S, Currie DW, Mangan J, Kaplan B, Goffard K, Salomon J, Casteel S, Mukasa A, Euhardy N, Ruiz A, Bautista G, Bailey E, Westergaard R, Gieryn D. Factors influencing risk for COVID-19 exposure among young adults aged 18–23 years—Winnebago County, Wisconsin, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1497–1501. doi: 10.15585/mmwr.mm6941e2external icon
Auld AF, Fielding K, Agizew T, Maida A, Mathoma A, Boyd R, Date A, Pals SL, Bicego G, Liu Y, Shiraishi RW, Ehrenkranz P, Serumola C, Mathebula U, Alexander H, Charalambous S, Emerson C, Rankgoane-Pono G, Pono P, Finlay A, Shepherd JC, Holmes C, Ellerbrock TV, Grant AD. Risk scores for predicting early antiretroviral therapy mortality in sub-Saharan Africa to inform who needs intensification of care: a derivation and external validation cohort studyexternal icon. BMC Med. 2020; 18(311). doi: 10.1186/s12916-020-01775-8external icon
Beeler Asay GR, Lam CK, Stewart B, Mangan JM, Romo L, Marks SM, Bamrah Morris S, Gummo CL, Keh CE, Hill AN, Thomas A, Macaraig M, St John K, J Ampie T, Chuck C, Burzynski J. Cost of tuberculosis therapy directly observed on video for health departments and patients in New York City; San Francisco, California; and Rhode Island (2017–2018)external icon. Am J Public Health. 2020 Nov 110(11):1696–1703. doi: 10.2105/AJPH.2020.305877external icon
Cegielski JP, Chan PC, Lan Z, Udwadia ZF, Viiklepp P, Yim JJ, Menzies D. Aminoglycosides and capreomycin in the treatment of multidrug-resistant tuberculosis: individual patient data meta-analysis of 12 030 patients from 25 countries, 2009–2016external icon. Clin Infect Dis. 2020 Oct. Epub ahead of print. doi: 10.1093/cid/ciaa621external icon
Collins JM, Stout JE, Ayers T, Hill AN, Katz DJ, Ho CS, Blumberg HM, Winglee K; Tuberculosis Epidemiologic Studies Consortium. Prevalence of latent tuberculosis infection among non-U.S.-born persons by country of birth—United States, 2012–2017external icon. Clin Infect Dis. 2020 Nov. Epub ahead of print. doi: 10.1093/cid/ciaa1662external icon
de Perio MA, Kobayashi M, Wortham JM. Occupational respiratory infectionsexternal icon. Clin Chest Med. 2020; 41:739–51. doi: 10.1016/j.ccm.2020.08.003external icon
Hill AN, Cohen T, Salomon JA, Menzies NA. High-resolution estimates of tuberculosis incidence among non-U.S.-born persons residing in the United States, 2000-2016.external icon Epidemics. 2020 Nov 10;33:100419. Online ahead of print. doi: https://doi.org/10.1016/j.epidem.2020.100419external icon
Willby M, Chopra P, Lemmer D, Klein K, Dalton TL, Engelthaler DM, Cegielski P, Posey JE; Global PETTS Investigators. Molecular evaluation of fluoroquinolone resistance in serial Mycobacterium tuberculosis isolates from individuals diagnosed with multidrug-resistant tuberculosisexternal icon. Antimicrob Agents Chemother. 2020: AAC.01663–20. Epub ahead of print. doi: 10.1128/aac.01663-20external icon
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Meredith Moore at mue1@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.