TB NOTES

TB Notes 3, 2019
Notes from the Director
Dear Colleague:

The past couple of months have been full of exciting updates from DTBE staff. In early October, DTBE released Reported Tuberculosis in the United States, 2018. The United States once again reported the lowest number of TB cases (9,025) and the lowest incidence rate on record (2.8 cases per 100,000). Although TB in the United States continues to decrease each year, the rate of decrease remains low. We hope this report will be a useful tool in informing and improving TB prevention and control activities. I want to especially thank all the state and local health departments throughout the United States whose staff collected and reported the data used in this publication.
As part of the ongoing TB Personal Stories series, DTBE released new stories featuring 4 four people diagnosed and treated for TB disease or latent TB infection. The stories of TB survivors can be a helpful motivational tool that TB programs and health care providers can use to encourage patients to complete treatment, and also a powerful tool to reach clinicians, policy makers, and members of the public less familiar with TB. DTBE would like to thank We Are TB, The National TB Controllers Association, and the Stop TB USA for their contributions to this project.
This fall has also been an opportunity to meet and collaborate with many of our partners and colleagues. DTBE staff recently traveled to Palau for the Pacific Islands TB Controllers Association (PITCA) Conference, where TB professionals, clinicians, and partners shared their cross-cutting and disease-specific expertise in TB. The Tuberculosis Trials Consortium (TBTC) held their 44th Semi-Annual Meeting October 15-17th in Atlanta. The meeting highlighted research on the diagnosis, clinical management, and treatment of latent TB infection and TB disease.
As we enter the final months of the year, I continue to be inspired by your dedication to the very important work you are doing every day in our efforts to eliminate TB. I hope all of you have a safe, enjoyable, and productive fall.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Communication Resources for the 2018 TB Surveillance Report
CEBSB created several resources to support the release of the 2018 TB surveillance report entitled Reported Tuberculosis in the United States, 2018, including an infographic and web graphics with highlights from the surveillance report. These resources support TB education and outreach to clinicians, health care agencies, and community organizations. TB control programs can also use a customizable infographic template to highlight state and local surveillance data.

This 2018 edition of Reported Tuberculosis in the United States marks a milestone as the first TB surveillance report to be published exclusively online, as part of CDC’s Digital First Initiative to help audiences discover, view, and share content more easily.
To learn more, visit the CDC TB website and follow us on Facebook and Twitter.

New Stories from the TB Personal Stories Series
CEBSB is proud to share the personal stories of four people diagnosed and treated for tuberculosis (TB) disease or latent TB infection. These new stories are part of DTBE’s ongoing TB Personal Stories series to help raise awareness about TB in the United States. You can view the video, print, and social media content for each story by visiting CDC’s TB Personal Stories website.
- Tenzin: Tenzin’s treatment for multidrug-resistant TB disease lasted for over 2 years. With the help of his local and state TB programs, Tenzin completed the long and challenging treatment process. Tenzin’s Story
- Kelcie: As someone who was tested and treated for latent TB infection to prevent the development of TB disease, Kelcie encourages others to do the same. Kelcie’s Story
- Kristi: Like many people, Kristi thought TB could only affect the lungs. She was surprised when she was diagnosed with TB in her eye. Kristi’s Story
Why Are These Stories Important?
Many TB patients report feeling isolated and stigmatized through their treatment. Hearing experiences from TB survivors can be a helpful motivational tool that TB programs and health care providers can use to encourage patients to complete treatment. Using patient stories in online, print, and social media content is also a powerful tool to reach clinicians, policy makers, and members of the public less familiar with TB.
Who Helped Make These Stories?
DTBE would like to thank We Are TB, The National TB Controllers Association, and the Stop TB USA for their contributions to this project. Over the past 5 years, these organizations have gathered a group of TB survivors to participate in a communication training workshop at the annual NTCA conference. DTBE has collaborated with survivors from these groups, as well as those identified from other TB programs, to feature in our TB Personal Stories series.
Stay Connected to TB Materials – Subscribe to Find TB Resources

Find TB Resources is a website that includes a searchable database of materials from numerous national and international organizations. Find TB Resources also delivers a monthly newsletter to keep subscribers informed about featured TB resources and other updates. To receive the monthly newsletter, please enter your email address at the following link.
If you have subscribed to the Find TB Resources newsletter in the past, please ensure that your subscription is still active by entering your email address here. The subscription is free of charge and will help you stay connected to TB materials!
2019 Pacific Islands TB Controllers Association (PITCA) Conference and TB Surveillance Training
On September 9–13, 2019, the Republic of Palau and Global TB Institute co-hosted the Pacific Islands TB Controllers Association (PITCA) Conference and TB Surveillance Training. This event assembled TB experts from throughout the Pacific Islands and U.S. to engage in learning and discussion on the management and control of TB and provided training and networking opportunities for TB professionals in the region. There were 127 participants at the conference, comprising of representatives from the six U.S.-affiliated Pacific Islands (USAPI) jurisdictions – American Samoa, Commonwealth of the Northern Mariana Islands, Federated States of Micronesia, Guam, Republic of Palau, and the Republic of the Marshall Islands. Partner agencies that also participated included the Australian Respiratory Council, CDC, Diagnostic Laboratory Services, Global TB Institute, National TB Controllers Association, United Nations Development Programme, and the World Health Organization.

The conference convened plenary sessions in the morning and breakout sessions in the afternoon, with separate tracks for laboratory staff, clinicians, nurses and outreach workers, and program managers.
The initial plenary session included a video from a TB survivor who discussed TB from the patient perspective and a keynote address from Dr. Stevenson Kuartei, a local physician who is a Senator in Palau’s Congress and who also was a former Minister of Health. Subsequent plenary sessions included presentations focused on the themes of TB treatment, prevention, and laboratory. Breakout tracks provided an opportunity to take a deep-dive into specific topics to address the training and education needs of the various disciplines in attendance.
The conference included a tour of the Belau National Hospital and the Communicable Disease Unit Clinic where TB patients receive care and treatment. There was also a site visit to the Southern Community Health Center on the island of Peleliu (population of 500), which was an opportunity for conference participants to better understand the infrastructure and provision of health services on this island. Clinicians had the opportunity to speak with a resident of Peleliu who is currently receiving treatment for Rifampin-resistant TB disease.

On September 16–18, 2019, the Republic of Palau hosted a TB Surveillance Training following the 2019 PITCA Conference. There were 27 participants at the training, which included representatives from five of the USAPI jurisdictions. Faculty from the CDC Division of Tuberculosis Elimination provided detailed instruction on how to interpret and properly complete each of the 43 variables of the 2020 RVCT and the Multidrug-Resistant (MDR) TB Supplemental Form. The classroom-based training included facilitated group discussions, interactive case studies, and opportunities for participants to exchange best practices. Educational resources and job aides were provided to improve the accuracy and completeness of reporting TB cases to CDC.
Reported Tuberculosis in the United States, 2018
Earlier this year, the Centers for Disease Control and Prevention (CDC) released provisional 2018 surveillance data on reported tuberculosis (TB) cases in the United States. The full report, entitled Reported Tuberculosis in the United States, 2018, is now available online.
Key findings:
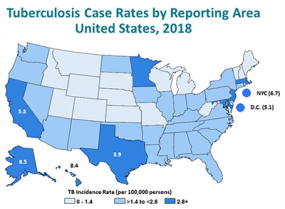
- There were 9,025 TB cases reported in the United States in 2018, which represents a 0.7% decrease from 2017.
- The overall annual TB incidence decreased to 2.8 cases per 100,000.
- People born outside of the United States continue to be disproportionately affected, largely because they have developed TB disease from longstanding latent TB infection that occurred in their country of origin.
The United States continues to have one of the lowest TB case rates in the world, and the 2018 case count represents the lowest number of TB cases on record. Still, too many people suffer from TB disease and our progress is too slow to eliminate TB in this century. Ending TB will require a dual approach of maintaining and strengthening current TB control priorities, while increasing efforts to identify and treat latent TB infection in high-risk populations.
Estimates of Recent Transmission, 2015-2018
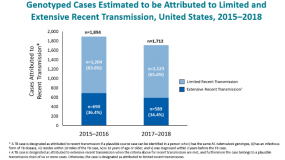
The Division of Tuberculosis Elimination (DTBE) has provided state- and county-level estimates of the numbers and proportions of genotyped TB cases attributed to recent transmission since the publication of Reported Tuberculosis in the United States, 2016. Using molecular surveillance data, a TB case is attributed to recent transmission if a plausible source case with a matching genotype and an infectious form of disease is identified in a patient who is 10 years of age or older, resides within a 10-mile geographic radius, and has a diagnosis within 2 years before the given case.
A stated goal of the provision of these estimates has been to help state and local TB programs monitor trends in recent transmission, which may assist with planning and prioritizing TB control activities. At the national level, the proportion of cases attributed to recent transmission has declined: 12.6% of genotyped TB cases were attributed to recent transmission during 2017-2018 compared with 13.7% for 2015-2016. DTBE epidemiologists are planning more detailed analyses to determine what might explain this apparent decline.
Additional information regarding DTBE’s recent transmission estimates is available here.
Submitted by Benjamin Silk, PhD, and Steve Kammerer, MBA
The National TB Program Objectives and Performance Targets for 2025
In September 2019, the Division of Tuberculosis Elimination released the national TB program objectives and performance targets for 2025. The national objectives include decreasing TB incidence rates for both US-born (0.4 cases per 100,000) and non-US—born populations (8.8 cases per 100,000), as well as for US-born minorities (1.0 case per 100,000) and children less than five years of age (0.1 cases per 100,000). The objectives incorporate key process indicators for patient management, laboratory reporting, contact investigations of infectious TB patients, and follow-up examination of immigrants and refugees who recently arrived in the United States with Class B notifications. The complete list of national objectives and targets can be found via https://www.cdc.gov/tb/programs/evaluation/indicators/default.htm
Indicators for measuring progress toward reaching the national objectives were established by the National TB Indicators Project (NTIP). Data reported to the National TB Surveillance System on the Report of a Verified Case of Tuberculosis (RVCT), the Aggregate Report for Program Evaluation (ARPE), and the Electronic Disease Notification (EDN) system are used in NTIP to calculate the indicators.
The 2025 national performance targets describe the level of success achieved by the top 10% of TB programs in the United State. To determine the targets, we used statistical models to find trends in NTIP data from 2010 through 2018. State and local TB programs with fewer than 150 TB cases from 2016–2018 were excluded. For each indicator, we developed a regression model to estimate the 90th percentile for each year, then used the fitted model to estimate the 90th percentile in the year 2025. The 90th-percentile values reflect the projected performance of the top 10% of TB programs in the United States in 2025.
TB program officials are encouraged to work closely with CDC Project Officers and Evaluation Consultants to conduct evaluation studies and to develop strategies to optimize performance to meet the targets.
Reported by Kai H. Young
Lessons Learned from TB Modeling through the NCHHSTP Epidemiologic and Economic Modeling Agreement
The first 5 years of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention’s epidemiologic and economic modeling cooperative agreement, NEEMA, concluded at the end of September 2019. When asked what they learned from their TB modeling experience, all three grantees determined based on model results that TB elimination is a distant goal using current tools and service provision levels; however, substantial progress can be achieved with extensive scale-up of targeted testing and treatment (TTT) of latent TB infection (LTBI) in non-US-born populations or with great improvements in global TB control.

The University of California’s Consortium for Applied Prevention Economics (CAPE) highlighted model findings that California TB cases could be cut in half by 2024 with a 4-fold increase in TTT of non-US-born residents and of persons with medical risks for progression. In addition, they estimated that full implementation of the US Preventive Services Task Force Recommendationsi or of the California TB Risk Assessmentii would also prevent half of projected TB cases in California within the first 10 years (over 9,500 TB cases). Models by each grantee showed that TTT of non-US-born persons would be most effective in making progress towards TB elimination, but that TTT of persons with HIV (e.g., those having the highest progression rates) would be most efficient.
Modelers from Johns Hopkins Bloomberg School of Public Health (JHSPH) emphasized that risk populations and their demographics differ by state, as does TB transmission and reactivation, and consequent cost effectiveness of TTT. Harvard University’s Prevention Policy Modeling Lab (PPML) remarked that comparing TB transmission models produced by each grantee applied to California data helped to enhance understanding of uncertainties around TB trends and the impact of interventions. Close collaboration with DTBE and state TB controllers was invaluable to modeling efforts, according to JHSPH.
NEEMA model results have corroborated CDC’s decision to focus on TTT for TB elimination, extended prior CDC modeling to 50 states and 20 counties, and produced TB case and LTBI prevalence point estimates and prediction intervals by population (including for some co-morbidities) that can assist TB programs in planning for TB elimination. The modeled scenarios produced powerful results and interactive tools that could be used for advocacy:
- Tabby 1, Harvard PPML US TB projections by scenario: https://ppmltools.org/tabby/external icon
- Tabby 2, Harvard PPML State-level TB projections by scenario: https://ppmltools.org/tabby2/external icon
- JHSPH State-level historical TB rates by risk population: http://www.modeltb.org/tbheteroinc/index.htmlexternal icon
- JHSPH TB projections by scenario for CA, FL, NY, TX: http://modeltb.org/fourState/pa10yr.htmlexternal icon
While models relied greatly on National TB Surveillance System (NTSS) data to which they were calibrated, additional data to reduce uncertainties are needed on population dynamics, LTBI prevalence and TB incidence progression rates by population, and on costs. DTBE encourages TB programs to publish programmatic experiences to document the impact and cost of activities. DTBE also encourages sharing of model results, specifically those on cost-effectiveness.
A current challenge is how to implement the modeled scenarios. Other key questions are:
- How can we best reach persons at highest risk for TB for targeted testing and treatment? In the context of electronic health records (EHRs), how can we identify populations having the highest risks of progression?
- How can we compare interventions such as better diagnostics and tools/algorithms to rule out TB disease using EHRs?
- How can we predict who will accept or decline LTBI treatment?
- In the United States, what do we need to do to accelerate TB elimination that might differ from other contexts? The United States is a leader in TB elimination efforts and often sets the standards for other countries.
Submitted by Suzanne Marks, MPH, MA
Atanaska Marinova-Petkova, DVM, MS, PhD and Calin Chiribau, MS, PhD have joined the Laboratory Branch as part of the Reference Laboratory Team (RLT). As senior biologists, both Atanaska and Calin will serve as part of the Molecular Detection of Drug Resistance (MDDR) service to perform testing for the rapid detection of resistance-associated mutations in Mycobacterium tuberculosis, expand capacity of the clinical service, and contribute to research studies to optimize molecular and growth-based assays for implementation in a CLIA regulated and ISO accredited environment.

Pictured here: Atanaska Marinova-Petkova, DVM, MS, PhD and Allison Kline, MS
Not pictured: Calin Chiribau, MS, PhD
Dr. Marinova-Petkova obtained her DVM degree in 2004 and went on to obtain a MS degree in Molecular Biology and Biotechnology in 2009 and PhD in Epizootiology and Infectious Diseases in Animals in 2012. She has extensive technical laboratory experience including virology, bacteriology, serology, molecular biology, and animal models. Dr. Marinova-Petkova completed a postdoctoral research appointment in the Department of Infectious Diseases at the St. Jude’s Research Hospital in Memphis before joining CDC as a Laboratory Advisor/Specialist in 2016. In 2017, she was selected as a Laboratory Leadership Service (LLS) fellow; a program in which she recently completed with CDC’s Zoonoses and Select Agent Laboratory in the Bacterial Special Pathogens Branch.
Dr. Chiribau comes to DTBE/LB from the Florida Department of Health, Bureau of Public Health Laboratories where he has worked in the mycobacteria laboratory at various levels since 2013, including General Laboratory Supervisor and Technical Lead. In these positions, Dr. Chiribau led the evaluation and implementation of molecular methods for rapid detection of drug resistance and new growth-based drug susceptibility testing and identification methods. Calin received his MS degree in 2002 in Molecular Genetics from Alexandru Iona Cuza University in Romania and his PhD in 2005 in Molecular Biology from Albert Ludwigs University in Germany. He then completed post-doctoral research fellowships at Emory University and Case Western Reserve University.
The DTBE/LB Applied Research Team also welcomed a new researcher in 2019. Allison Kline, MS, recently joined the team as an ORISE Fellow. This fellowship will allow Allison to train with LB immunologists on state-of-the-art technologies to contribute to expanding Mycobacterium tuberculosis research in two main areas, host-directed therapies and drug resistance. Ms. Kline received her BS in Environmental Science from the University of North Carolina at Chapel Hill in 2016 and her MS in Environmental Health from University of Washington School of Public Health in 2019.
DTBE/LB is proud to have these individuals join our staff of hard working and innovative scientists.
Submitted by Stephanie Johnston, MS

TBTC Study 37: Assessment of the Safety, Tolerability, and Effectiveness of Rifapentine Given Daily for LTBI (ASTERoiD) (ClinicalTrials.gov Identifier: NCT03474029) tests a shorter, simpler latent TB infection regimen. The trial aims to compare a 6-week regimen of daily self-administered rifapentine with 2 or 3 rifamycin-based comparator regimens (3HP, 4R, 3HR), for the treatment of drug-sensitive latent TB infection. It seeks to enroll at least 3,400 persons. The trial opened for enrollment July 26, 2019: six participants have been enrolled as of mid-August 2019. Four TBTC sites are enrolling, and additional sites are expected to open soon.
TBTC Study 35: Phase I/II Dose Finding and Safety Study of Rifapentine and Isoniazid in HIV-Infected and HIV-Uninfected Children with Latent Tuberculosis Infection (ClinicalTrials.gov Identifier: NCT03730181) aims to open in the last quarter of 2019. Study 35 will evaluate tolerability and pharmacokinetics of a new child-friendly formulation of isoniazid and rifapentine in children aged 0-12 years, at 2 sites in South Africa. It will enroll about 72 participants.
TBTC Study 31/ACTG A5349: Rifapentine-containing Tuberculosis Treatment Shortening Regimens (ClinicalTrials.gov Identifier: NCT02410772) completed enrollment of 2,516 participants in October 2018; follow-up is continuing with initial results expected in the second half of 2020. This large phase 3 trial has been a successful collaboration between CDC’s TBTC and NIAID/Division of AIDS’ Adult AIDS Clinical Trials Group (ACTG). Several significant sub-studies are nested within the trial, which is expected to provide substantial information on drug pharmacokinetics, biomarkers, and adherence effects.
DTBE has been partnering with the TB Control Program in New York City to implement a randomized trial of electronic Directly Observed Therapy (DOT). The trial compares smartphone-based video DOT (either real-time or recorded) with traditional in-person DOT in new TB cases (ClinicalTrials.gov Identifier: NCT03266003). Enrollment will conclude at the end of October, and data collection efforts will conclude in January 2020. In addition to assessing adherence with each modality, the trial includes an economic sub-study to evaluate relative costs of the 2 approaches to DOT.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email John Parmer at bkz8@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.
Armstrong LR, Winston CA, Stewart B, Tsang CA, Langer AJ, Navin TR. Changes in tuberculosis epidemiology, United States, 1993–2017. Int J Tuberc Lung Dis 2019;23:797–804.
Bonney W, Price SF, Miramontes R. Design and implementation of data exchange formats for molecular detection of drug-resistant tuberculosis. AMIA Jt Summits Transl Sci Proc 2019;2019:686–95. (Bonny, Public Health Informatics Fellowship Program, assigned to DTBE).
Cowger TL, Wortham JM, Burton DC. Epidemiology of tuberculosis among children and adolescents in the USA, 2007–17: an analysis of national surveillance data. Lancet Public Health 2019;pii:S2468–2667(19)30134–3. Epub ahead of print.
Lienhardt C, Vernon AA, Cavaleri M, Nambiar S, Nahid P. Development of new TB regimens: harmonizing trial design, product registration requirements, and public health guidance. PLoS Med 2019;16:e1002915.
Marks SM, Woodruff RY, Owusu-Edusei K, Asay GRB, Hill AN. Estimates of testing for latent tuberculosis infection and cost, United States, 2013. Public Health Rep 2019:33354919862688. Epub ahead of print. (Also from OD [Owusu-Edusei]).
Mase SR, Samron R, Ashkin D, Castro KG, Ryan S, Seaworth B, Chen L, Lardizabal A, Tuckey D, Khan A, Posey DL, Chappelle C, Temesgen Z. Tuberculosis regional training and medical consultation centers in the United States: characteristics, outcomes, and quality of medical consultations, June 1, 2010–May 31, 2014. J Clin Tuberc Other Mycobact Dis 2019;17:100114.
Sharling L, Marks SM, Goodman M, Chorba T, Mase S. Rifampin-resistant tuberculosis in the United States, 1998–2014. Clin Infect Dis 2019;pii:ciz491. Epub ahead of print.
Shrestha S, Cherng S, Hill AN, Reynolds S, Flood J, Barry PM, Readhead A, Oxtoby M, Lauzardo M, Privett T, Marks SM, Dowdy DW. Impact and effectiveness of state-level tuberculosis interventions in California, Florida, New York and Texas: a model-based analysis. Am J Epidemiol 2019;pii:kwz147. Epub ahead of print.
Sosa LE, Njie GJ, Lobato MN, Morris SB, Buchta W, Casey ML, Goswami ND, Gruden M, Hurst BJ, Khan AR, Kuhar DT, Lewinsohn DM, Mathew TA, Mazurek GH, Reves R, Paulos L, Thanassi W, Will L, Belknap R. Tuberculosis screening, testing, and treatment of U.S. health care personnel: recommendations from the National Tuberculosis Controllers Association and CDC, 2019. Am J Transplant 2019;19:2383–7.
Venkatappa TK, Punnoose R, Katz DJ, Higgins MP, Banaei N, Graviss EA, Belknap RW, Ho CS; Tuberculosis Epidemiologic Studies Consortium. Comparing QuantiFERON-TB Gold Plus with other tests to diagnose Mycobacterium tuberculosis infection. J Clin Microbiol 2019;pii:JCM.00985–19. Epub ahead of print. (Punnoose, with Northrop Grumman, assigned to DTBE).
Wilson JW, Nilsen DM, Marks SM. Multidrug resistant tuberculosis in patients with HIV: management considerations within high-resourced settings. Ann Am Thorac Soc 2019. Epub ahead of print.
Zimba O, Tamuhla T, Basotli J, Letsibogo G, Pals S, Mathebula U, Mathoma A, Serumola C, Ramogale K, Boyd R, Tran T, Finlay A, Auld A, Date A, Alexander H, Chihota V, Agizew T. The effect of sputum quality and volume on the yield of bacteriologically-confirmed TB by Xpert MTB/RIF and smear. Pan Afr Med J 2019;33:110.
i U. S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Bauman L, Davidson KW, et al. Screening for Latent Tuberculosis Infection in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(9):962-9.
ii California Department of Public Health. California Adult TB Risk Assessment. https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/TBCB-CA-TB-Risk-Assessment-and-Fact-Sheet.pdf