TB NOTES

TB Notes 32018
Notes from the Director
Dear Colleague:

This past spring, the Division of Tuberculosis Elimination (DTBE) staff have been involved in a variety of events. DTBE hosted a TB Expert Network Conference in late April with the TB Centers of Excellence for Training, Education, and Medical Consultation (TB COEs). The topic of the conference was What’s New on the Menu for TB Treatments? A Discussion of Drugs for a Complicated U.S. TB Patient.
DTBE representatives attended a two-day meeting, Primary Care and Public Health: Partners in Prevention Meeting, in Atlanta May 7-8. Experts from clinical provider associations exchanged information about ways to enhance screening and linkage to care service. DTBE welcomed field staff to Atlanta May 7-10 for its Field Services Branch meeting. Field staff and headquarters staff received updates on a variety of topics, and shared information and ideas.
The Tuberculosis Trials Consortium (TBTC) held their 41st Semi-Annual Meeting May 8-10 in Atlanta. The meeting highlighted research on the diagnosis, clinical management, and treatment of latent TB infection and TB disease. The 9th annual Tuberculosis Epidemiologic Studies Consortium (TBESC) meeting was held May 10-11 in Atlanta. The meeting highlighted current and planned TBESC studies that encompass elements of latent TB Infection testing, treatment, program evaluation, and opportunities to increase uptake of testing and treatment, such as working with community providers. These meetings have given us an opportunity to connect with many important partners, set research priorities, and identify activities for us to focus on for the remaining of the year and beyond.
Congratulations to Dr. Suraj Sable of the Laboratory Branch in DTBE, who won first place for best poster in the safety category at the CDC Laboratory Science Symposium. Dr. Sable presented data on the standardization and validation of an aerosol infection model for Mycobacterium tuberculosis. This work established the foundation for future studies on novel strategies for the treatment of drug-resistant and susceptible tuberculosis using the mouse model.
As we enter the last half of the year, I continue to be inspired by your dedication to the very important work you do every day in our efforts to eliminate TB. I hope all of you have a safe, enjoyable, and productive summer.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
New Branch Chief

Nickolas (Nick) DeLuca, PhD, is joining DTBE as the Chief of the Communications, Education, and Behavioral Studies Branch (CEBSB). Dr. DeLuca previously served as the Associate Director for Communication Science in the Center for Global Health (CGH). There, he led a variety of important actions including the development and implementation of many new communication products, extensive updates to web-based and social media materials, delivery of emergency and risk communication training to countries throughout the world, and media relations with a variety of domestic and international media outlets.
From 2012 to 2016, Dr. DeLuca served as the Chief of the Prevention Communication Branch in the Division of HIV/AIDS Prevention. In that role, he oversaw science-based HIV communications programs and campaigns promoting HIV awareness, testing, prevention, and services for the public, persons at risk for and living with HIV, as well as for health care professionals treating and caring for persons with HIV. From 2008 to 2011, Dr. DeLuca was the Prevention Advisor with CDC’s Global AIDS Program in Namibia, where he managed HIV and TB prevention, communications, education, and intervention programs.
Dr. DeLuca began his CDC career in CEBSB in 1997, and he became Team Lead for the Education, Training, and Behavioral Studies Team in 2002. As team lead, he oversaw a variety of behavioral science research activities and developed numerous TB-related education, training, and communication products. He is an author on several scientific publications from his work in DTBE. Dr. DeLuca received his PhD in Health Education and Health Promotion from the University of Alabama at Birmingham.
Submitted by Sloane Bowman, MPH, DTBE
Mark your calendar for the 2018 TB Education and Training Network and TB Program Evaluation Network Conference
The 2018 TB Education and Training Network (ETN) and the TB Program Evaluation Network (PEN) Conference, Breaking through Barriers to Achieve TB Elimination, will be held September 18-20 in Decatur, Georgia. This year’s conference will highlight common aspects of TB education, training, and evaluation. The opening plenary will focus on how TB education, training, and evaluation play key roles in removing the barriers preventing elimination of TB in the United States. Additional plenary sessions will include the latest advances in latent TB infection surveillance and reporting, social media, and presentations of some of the best state and local TB education, training, and evaluation projects in the country.
There will be a wide variety of ETN and PEN skills-based workshops and informational presentations. Topics for these workshops and presentations include designing data collection instruments, engaging and educating primary care providers on latent TB infection, and the importance of health literacy. The conference also offers wonderful opportunities to network with colleagues.
The call for conference abstracts is open and authors of winning submissions will share and discuss their work during a poster session. Additionally, two ETN abstracts and two PEN abstracts will be chosen for oral presentations. Appropriate topics for TB ETN posters include activities associated with the systematic health education process, such as needs assessment, development, pilot testing, implementation, and assessing effectiveness. Appropriate topics for TB PEN posters include methods related to systematic program evaluation, such as capacity building, engagement in monitoring and evaluation, and sharing lessons learned from evaluation.
Send your completed abstract submission form to:
- TB ETN abstracts: htz7@cdc.gov
- TB PEN abstracts: zex5@cdc.gov
The deadline for abstract submission is June 29, 2018.
The conference venue is the Marriot Courtyard Decatur, 130 Clairemont Avenue, Decatur, Georgia 30030. To reserve a room at the conference rate, be sure to make your reservation before August 27. You may reserve a room online at Book your group rate for CDC Division of TB Elimination ETN PEN 2018 Conference or by calling Marriott Central Reservations at 1-888-236-2427 or Courtyard Decatur Front Desk: 404-371-0204. Be sure to identify yourself as an attendee of the CDC Division of TB Elimination ETN PEN 2018 Conference. The conference registration deadline is August 27. Note: there is no registration fee to attend the conference. Please submit your conference registration form to tbetn@cdc.gov. For additional information, please visit the TB ETN conference website.
Submitted by Peri Hopkins, MPH, DTBE
TB Centers of Excellence for Training, Education, and Medical Consultation Needs Assessment
The TB Centers of Excellence for Training, Education, and Medical Consultation (TB COEs) will be conducting a needs assessment for TB professionals this spring/summer to identify the training, education, and medical consultation gaps, priorities, and existing resources in their regions. The methods will include an online survey as well as key informant interviews with TB program staff. The online survey is tentatively scheduled to be released at the end of May.
Results from the needs assessment will guide the development of the TB COEs’ work plans and strategies to engage their respective regions. Additionally, DTBE will use the results from the needs assessment to develop a national picture of training, education, and medical consultation needs. We encourage TB Program Professionals to take the time to complete this assessment to help the TB COEs identify your needs.
Submitted by Sarah Segerlind, MPH, DTBE
Case Study: Instagram Story

In honor of World TB Day, DTBE took over CDC’s Instagram account. An Instagram takeover involves assuming temporary control of someone else’s Instagram account and sharing content with their audience. A unique feature we utilized for the takeover was an Instagram Story, which lets you share the moments of your day. As you share multiple photos and videos, they appear together in a slideshow format- your story. The Story vanishes after 24 hours, and the content will not appear on the profile grid or in the main Instagram feed.
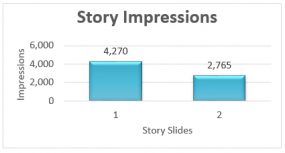
Our Instagram Story highlighted the great work of our field staff who are embedded in state and local health departments. This Story promoted the assistance DTBE provides to state and local TB control programs by helping to find, treat, and prevent TB. The Instagram Story had 7,035 impressions (total number of times the story has been seen).
CDC’s Instagram account currently has 74K followers. That reach allowed us to broaden our audience and highlight TB to a new audience that may not follow DTBE’s social media platforms. An Instagram takeover is a great way to utilize existing resources to expand reach and build brand awareness. You can also follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates here.
Submitted by Sloane Bowman, MPH, DTBE
Teresa Goss Hutchins Retirement
Teresa Goss Hutchins retired on April 28, after 27 years of federal service. Teresa began her federal career in 1991 as an administrative assistant with the Internal Revenue Service. In 1998, Teresa found her home at CDC in DTBE, after her mother (also a CDC employee) encouraged her to join the agency. Teresa worked as a Management and Program Analyst for both the Division’s Surveillance, Epidemiology, and Outbreak Investigation Branch and the Field Services Branch.

For the last 16 years, Teresa has served as a Training Specialist with the Communications, Education, and Behavioral Studies Branch. Teresa oversaw the printing, publication, and distribution of the extensive library of DTBE products, working closely with state and local TB control programs to ensure they had access to publications. She coordinated trainings, prepared materials for countless DTBE initiatives, and completed many continuing education applications. Teresa handled the logistics for the annual TB Program Managers’ Course and biannual TB ETN Conference. She also managed the TB ETN member database and TB TRAIN.
Teresa was always the first to welcome attendees to any conference or training, greeting them with a smile and providing a perfectly organized information packet of everything they needed. For people inside and outside CDC, whenever a question came up about TB training, the answer was “let’s ask Teresa!” No problem was ever too big or too small for Teresa to tackle.
Teresa’s giving spirit and dedication to helping others is evident in her professional and personal life. From tracking down information for a partner, collecting donations to purchase holiday gifts for foster children, or building TB-themed scarecrows for the Botanical Gardens – Scarecrows in the Garden Event, you can always count on Teresa to be the first to volunteer to help. We wish Teresa, her husband Todd, and her beloved dog, Bruizer, all the best in the future!
Submitted by Maria Fraire Sessions, MPH, CHES, DTBE
Surveillance for TB Elimination Management System (STEMS)
In keeping with the DTBE’s focus on improvement of testing, treatment, and treatment completion for latent TB infection in high-risk populations, the Tuberculosis Epidemiologic Studies Consortium (TBESC) has developed a latent TB infection surveillance and case management system for use by local jurisdictions. STEMS, developed in collaboration with Northrop Grumman (NG), is a secure, web-based system currently used for research purposes by all TBESC health department sites. The real-time system permits evaluation and tracking of all patients tested for latent TB infection. It is free to all users.
Since January 2017, when STEMS was first deployed, improvements including report functions that generate (1) lists of past or future appointments, (2) latent TB infection tests by date and reason evaluated, and (3) a downloadable data shell that can be populated by minimum data elements required to produce a TB cascade to cure for high-risk populations, have been added. A contact investigation (CI) module and a case management function for TB cases are under development.

The CI module was demonstrated at the TBESC meeting, on May 10-11, 2018. We will also conduct a hands-on training for attendees at the TB-Program Evaluation Network (TB PEN) meeting in Atlanta, September 18-20, 2018. Local areas interested in using STEMS should discuss the opportunity with their state health department and CDC state public health advisor before contacting Kumar Batra, Technical Program Manager (atz3@cdc.gov) for access to STEMS.
Submitted by Kiana Woods, MBA, DTBE
Diagnostic Mycobacteriology Workshop and Laboratory Professionals Week

During the week of April 24, the DTBE Laboratory Branch hosted the annual Mycobacterium tuberculosis: Diagnostic Principles and Procedures Workshop at CDC. Twenty laboratorians selected from states and countries across North America participated in this four-day course. Topics related to laboratory risk assessment, biological safety cabinet practices, specimen processing and isolation, false-positive and false-negative results prevention, mycobacteriology test methods, drug susceptibility testing, laboratory performance indicators, validation principles, molecular testing including whole genome sequencing, and TB epidemiology. Participants performed real-time PCR during a hands-on laboratory session and contributed to individual and group case study exercises. Participants also enjoyed a presentation titled, Beyond the Laboratory Walls: Enhancing Your Integrated System, presented by Jessica Gentry, Tuberculosis Laboratory Supervisor at the Indiana State Department of Health. Jessica spoke about the importance of building relationships with the public health laboratory, TB program, and external laboratory partners. The next TB Diagnostic Principles and Procedures Workshop will be held in spring of 2019 with applications due in late 2018.
Medical Laboratory Professionals Week (April 22–28, 2018) was celebrated during the workshop. Medical laboratory professionals have been recognized during the last week of April since 1975 with the week focused on providing recognition to the essential contributions of laboratory professionals. Laboratory professionals play an important role in diagnosis, prevention, and treatment of disease as well as with collaborations with other medical professionals. For more information about Medical Laboratory Professionals Week, please visit any of the following links:
- The Division of Laboratory Systems (DLS) in the Center for Surveillance, Epidemiology, and Laboratory Services (CSELS):
Medical Laboratory Professionals Week - American Society for Clinical Pathology: Lab Week 2018
Monica Youngblood, MPH, M (ASCP), DTBE
The Latest News from the TB Trials Consortium (TBTC)
TBTC Study 31 (Rifapentine-containing treatment shortening regimens for pulmonary tuberculosis: A randomized, open-label, controlled phase 3 clinical trial) continues to enroll. As of April 9, 2018, the study had 1,887 participants, 75% of target enrollment.

TBTC Study 37 (Assessment of the Safety, Tolerability, and Effectiveness of Rifapentine given Daily for latent TB infection). The goal of this study is to evaluate novel ultra-short (6 weeks) latent TB infection treatment regimen. This is a collaboration with the TB Epidemiologic Studies Consortium and the Medical Research Council (United Kingdom) for a 3,400-patient, open-label, randomized controlled trial. The CDC IRB has approved the study and it is now on the clinical trials website. Participating sites are working on obtaining local IRB approvals. TBTC aims to start enrollment into the trial in the late summer early fall of 2018.
TBTC was a runner up for the Kochon Prize 2017 for their work on TB drugs. The Kochon Prize has been given annually for the past 11 years to individuals and/or organizations that have made a highly significant contribution to ending TB. The semi-annual TBTC meeting took place on May 8-10, 2018 at the Crown Plaza Ravinia hotel in Atlanta.
CROI Report
The AIDS Clinical Trials Group (ACTG) presented One Month of Rifapentine/Isoniazid to Prevent TB in People with HIV: Brief-TB/A5279 at the 2018 Conference on Retroviruses and Opportunistic Infections (CROI). The results conclude that 1HP provides a highly effective, ultra-short course regimen for the prevention of TB in people with HIV.
Submitted by Barbara DeCausey, MPH, MBA, DTBE
This summary describes the recently published manuscript, Development of a Surveillance Definition for United States–Mexico Binational Cases of Tuberculosis, by Rachel Yelk Woodruff, Mark Miner, Roque Miramontes in collaboration with Juli Bettridge, Miguel Escobedo, Diana Fortune, Maria Galvis, Lupe Gonzalez, Eric Hawkins, Paula Kriner, Kathleen Moser, Maria Rodriquez, Neha Shah, Cynthia Tafolla, and Courtney Yuen.
Successful treatment of persons with TB who cross international borders during their infectious or treatment periods requires significant collaborative efforts among TB programs in more than one country. However, due to the lack of a uniformly applied surveillance definition for binational TB cases, there is currently no available data to quantitatively describe the extent of the increased staff time and resources binational cases require of United States TB control programs or epidemiologic trends in binational cases over time. Furthermore, the lack of standardized identification of binational TB cases makes it difficult for programs to evaluate and improve binational TB control activities.
The objective of this study was to develop a surveillance definition for binational (United States–Mexico) cases of TB to assess the burden on US TB program resources.
We collaborated with state and local TB program staff members in the United States to identify characteristics associated with binational cases of TB. We collected data on all cases of TB from 9 pilot sites in 5 states (Arizona, California, Colorado, New Mexico, and Texas) during January 1–June 30, 2014, that had at least 1 binational characteristic. A workgroup of U.S. state, local, and federal partners reviewed results and used them to develop a practical surveillance definition.
The Surveillance Definition for Binational Tuberculosis Cases Workgroup recommended the following definition for a binational case of TB:
- Tuberculosis disease in a patient who crossed the border into the U.S. from Mexico during treatment, or
- Tuberculosis disease in a patient who was referred to a US-funded, binational TB program for treatment continuity (i.e., a patient who was being treated in the United States but it was known that he or she would cross the border to Mexico), or
- Tuberculosis disease in a patient who does not physically reside in the U.S. and did not receive treatment in the U.S. but received treatment in Mexico through a US-funded, binational program (i.e., funding for patient’s treatment in Mexico came from the United States; unknown if patient will ever cross the border to the U.S.).
The pilot sites reported 87 cases of TB with at least 1 binational characteristic during the project period. Applying the new proposed definition, 39 of 87 pilot cases of TB (45%) met the definition of binational.
Input from partners who were responsible for the care and treatment of patients who cross the United States–Mexico border was crucial in defining a binational case of TB. Our definition will allow local and national analysis of trends and evaluation of activities, which will ultimately lead to improvement in the care, and management of binational TB patients. The full manuscript is available in Sage Journals Public Health Reports.
Submitted by Rachel Yelk Woodruff, MPH, DTBE
April 2018
Beavers SF, Pascopella L, Davidow AL, Mangan JM, Hirsch-Moverman YR, Golub JE, Blumberg HM, Webb RM, Royce RA, Buskin SE, Leonard MK, Weinfurter PC, Belknap RW, Hughes SE, Warkentin JV, Welbel SF, Miller TL, Kundipati SR, Lauzardo M, Barry PM, Katz DJ, Garrett DO, Graviss EA, Flood JM; Tuberculosis Epidemiologic Studies Consortium. Tuberculosis mortality in the United States: epidemiology and prevention opportunities. Ann Am Thorac Soc 2018. Epub ahead of print.
Green KD, Biswas T, Pang AH, Willby MJ, Reed MS, Stuchlik O, Pohl J, Posey JE, Tsodikov OV, Garneau-Tsodikova S. Acetylation by Eis and deacetylation by Rv1151c of Mycobacterium tuberculosis HupB: biochemical and structural insight. Biochemistry 2018;57:781–90.
Poghotanyan G, Feng Z, Glasser JW, Hill AN. Constrained minimization problems for the reproduction number in meta-population models. J Math Biol 2018. Epub ahead of print.
Shah NS, Westenhouse J, Lowenthal P, Schecter G, True L, Mase S, Barry PM, Flood J. The California Multidrug-Resistant Tuberculosis Consult Service: a partnership of state and local programs. Public Health Action 2018;8:7–13.
Weiner M, Gelfond J, Johnson-Pais TL, Engle M, Peloquin CA, Johnson JL, Sizemore EE, Mac Kenzie WR; Pharmacokinetics/Pharmacodynamics Group of Tuberculosis Trials Consortium. Elevated plasma moxifloxacin concentrations and SLCO1B1 g.-11187>A polymorphism in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2018;pii:AAC.01802–17. Epub ahead of print.
May 2018
Reaves EJ, Shah S, France AM, Morris SB, Bradley H. In reply: latent tuberculous infection testing among HIV-infected persons in clinical care. Int J Tuberc Lung Dis 2018;22:468–9. (Also from DHAP [France and Bradley]).
Stewart RJ, Tsang CA, Pratt RH, Price SF, Langer AJ. Tuberculosis—United States, 2017. MMWR Morb Mortal Weekly Rep 2018; 67:317–23.
Woodruff RSY, Miner MC, Miramontes R. Development of a surveillance definition for United States-Mexico binational cases of tuberculosis. Public Health Rep 2018;133:155–62.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage.
If you would like to submit an article or update in TB Notes, please email Sloane Bowman at wnv2@cdc.gov