Hepatitis C Surveillance Guidance
- Background
- Cases and Clusters of Potential Public Health Importance
- Interpretation of Laboratory Test Results
- Recommended Reportable Laboratory Markers
- Case Reporting and National Notification
- Surveillance of Acute and Chronic Hepatitis C
- Surveillance of Hepatitis C During Pregnancy and Perinatal Hepatitis C
Background
Hepatitis C is a disease caused by the hepatitis C virus (HCV) that can be a short-term illness, but for more than one-half of people who become infected, it can become a long-term, chronic infection (74). HCV is one of the most common bloodborne pathogens in the United States (75-77). It is highly infectious and can survive on dry surfaces and equipment for up to 6 weeks, resulting in a longer period for potential transmission than for other bloodborne pathogens (e.g., HBV and HIV) (78). HCV is most efficiently transmitted through blood-to-blood contact or through percutaneous exposure to blood (75). Injection drug use (IDU) is the most common risk behavior reported for HCV infection (3). Among people who inject drugs (PWID), sharing of needles and syringes is most strongly associated with hepatitis C (79). Populations at highest risk for having hepatitis C include PWID, HIV-positive MSM, people with a history of incarceration, and people born during 1945–1965 (baby boomer birth cohort) (80). Approximately 75%–85% of people with acute hepatitis C are not symptomatic (81-83); as such, measuring the true burden of disease is difficult.
The epidemiology of hepatitis C in the United States has changed substantially. After decades of decline in acute hepatitis C incidence, rates began increasing in 2010. Increases in both acute and chronic hepatitis C, associated with IDU, shifted from people born during 1945–1965 to a younger population (15) that is typically non-Hispanic White. People of American Indian/Alaska Native and non-Hispanic Black race/ethnicity also experience disproportionately high rates of infection and mortality (3). Though these groups are disproportionately affected by hepatitis C at the national level, disparities vary among jurisdictions.
During January 2017–March 2020, the US prevalence of chronic hepatitis C, as measured by the presence of HCV RNA in blood, was estimated to be 0.9% (95% CI, 0.5%–1.4%), representing approximately 2.2 million adults (2). Jurisdictional-specific estimates would be more useful for program planning and evaluation at the state, territorial, and local level. Without treatment, 20%–30% of people with chronic hepatitis C progress to cirrhosis over a 25–30-year period (84).
In addition, nearly one-third of people with hepatitis C are unaware of their infection status and can unknowingly transmit the virus to others (2). Hepatitis C- and liver cancer-associated death rates were highest among decedents who were born during 1945–1965 (85). Hepatitis C-associated death rates have declined each year since 2013 (3). The decline in the national hepatitis C-associated death rate is likely due to the evolving epidemiology of hepatitis C, as people disproportionately affected by hepatitis C have died in earlier years and more people are being cured with DAA drugs.
Children born to HCV RNA-positive gestational parents are also at risk for hepatitis C. The rate of perinatal HCV transmission is approximately 5.8% in HCV RNA-positive/HIV-negative mothers and 10.8% in HCV RNA-positive women who have HIV coinfection (86). DAA treatment is recommended for people with childbearing potential before considering pregnancy to reduce HCV transmission to any future offspring (87). There are no treatment recommendations during pregnancy; however, it may be considered on a patient-level basis after a physician-patient counsel about the possible risks and benefits (87).
There is no vaccine to prevent hepatitis C. Therefore, the best way to prevent infection is by avoiding behaviors that can transmit the virus, such as sharing drug injecting equipment (e.g., needles, syringes, works, and cookers). Research has shown that maintenance MOUD can also be effective in reducing HCV transmission among PWIDs. If exposure is suspected to have occurred, getting tested and seeking treatment can prevent complications related to HCV infection and interrupt transmission.
In 2011, DAA therapies were approved for the treatment of chronic hepatitis C (88). In 2014, therapies became all-oral and highly effective (89). In 2019, as part of the “test and treat” strategy, the AASLD/Infectious Diseases Society of America (IDSA) updated their hepatitis C treatment guidance to recommend initiating treatment in patients with acute hepatitis C upon first diagnosis without waiting for spontaneous resolution to occur (87). In addition, some DAAs are now approved by FDA for children ≥3 years of age and are recommended by AASLD/IDSA for treatment (87).
Recommendations for universal hepatitis C screening were released by the CDC in 2020 (90). They recommend that all adults >18 years of age be tested at least once and that all pregnant people be tested during each pregnancy, except in settings where the prevalence is known to be less than 0.1%. All people with risk behaviors or exposures should be tested for hepatitis C, with periodic testing while risk behaviors or exposures persist. In addition, any person who requests hepatitis C testing should receive it, regardless of disclosure of risk, because many people might be reluctant to disclose stigmatizing information. If the HCV RNA prevalence is unknown in a setting, health care providers should test all adults and pregnant people for hepatitis C until the HCV RNA prevalence is determined to be less than 0.1%, at which point providers can screen at their discretion (90).
To determine the HCV RNA prevalence, health care providers and program directors are encouraged to consult with their state, territorial, or local HDs or with CDC to determine a method for calculating the HCV RNA baseline prevalence in their setting. Approximately 59% of people positive for anti-HCV are positive for HCV RNA (90). Therefore, as a general guide, an estimated 507 randomly selected patients in any sized setting would need to be tested for anti-HCV in order to detect an anti-HCV prevalence of <0.17%, corresponding to an expected HCV RNA prevalence of 0.1% (90).
The purpose of this document is to provide jurisdictional guidance to implement and improve hepatitis C surveillance, including reporting requirements, collection of relevant laboratory data, and case investigation. Given that current systems for the surveillance and follow-up of hepatitis C cases differ by jurisdiction, the standards outlined in this document are designed to provide models for best practices, recognizing that not every jurisdiction can meet those standards with available resources.
Cases and Clusters of Potential Public Health Importance
Jurisdictions should review and analyze hepatitis C data regularly to identify cases and clusters of hepatitis C that merit further investigation. When resources are limited, these should be prioritized for investigation according to degree of public health importance. The following are examples of high priority cases and clusters:
- People of childbearing age who are or have the potential to become pregnant, indicating the potential risk for perinatal transmission.
- Children ≤36 months of age, indicating possible perinatal transmission.
- People in age and demographic groups among whom infection might be acute due to recent transmission. This includes people
- <40 years of age (population experiencing greater increase in acute hepatitis C incidence) and
- >70 years of age (possible health care-associated transmission).
- People receiving hemodialysis with evidence of acute hepatitis C (including test conversions).
- People who do not have typical risk behaviors for hepatitis C (e.g., IDU) but who have evidence of acute infection (including test conversions). These people should be investigated to identify other potential causes of HCV transmission (e.g., exposure through health care). Information on investigation of health care-associated outbreaks is available through CDC’s DVH.
- People with other indicator(s) of possible acute or recent infection, including those
- with elevated ALT or total bilirubin levels;
- with current or recent IDU history;
- who were tested at locations where people at high risk for acute infection are typically seen (e.g., STI and HIV clinics, SSPs, correctional facilities, and MAT centers); or
- who were in a residential facility or custodial care (including long-term care or correctional facilities) for ≥6 months prior to the onset of clinical signs.
Interpretation of Laboratory Test Results
The two tests used primarily for hepatitis C screening and diagnosis are an antibody test (often an immunoassay) and an RNA test (NAT), respectively (94). A description of hepatitis C laboratory markers can be found in Appendix B. Figure 4-1 describes the typical serologic course of HCV infection (95).
Figure 4-1. Typical serologic course of hepatitis C virus infection
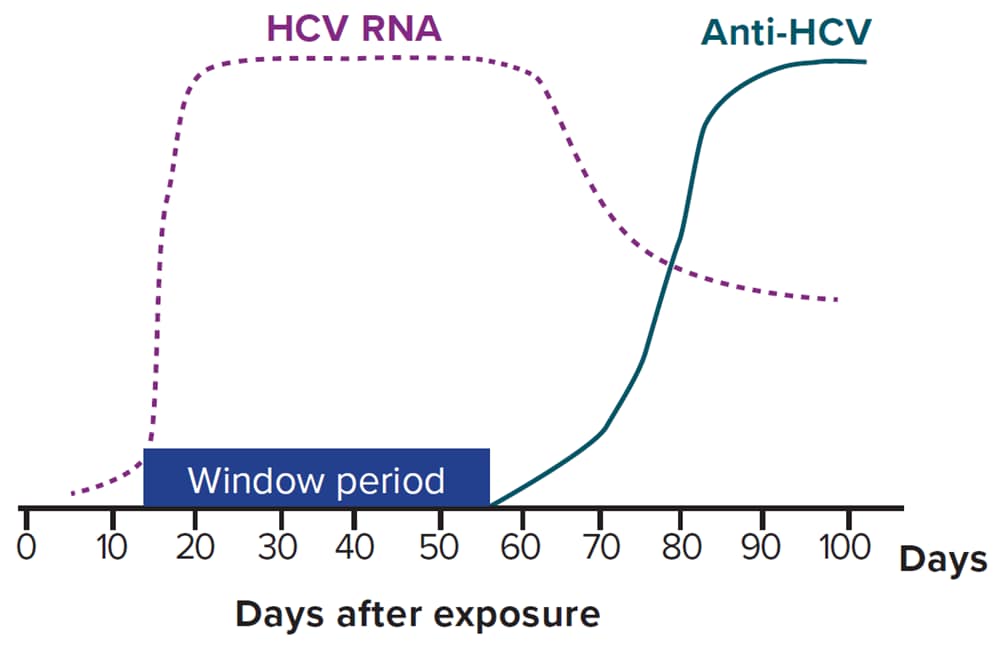
Downloads of this figure: PDF | PPT
In 2013, CDC provided updated guidance on the recommended testing sequence for identifying current hepatitis C (96). Hepatitis C testing should be initiated with an anti-HCV screening test, and if positive, an HCV RNA test should be performed. In settings serving high-risk populations (e.g., SSPs), rapid anti-HCV testing (also called point-of-care testing) can be used in lieu of laboratory-based anti-HCV testing to deliver results to the patient at the time of visit. For people who tested anti-HCV positive through rapid screening, an on-the-spot blood draw to be sent for HCV RNA testing should be performed or a referral and/or evaluation for HCV RNA testing should be provided. For blood draws collected for anti-HCV testing, all positive specimens should reflex to HCV RNA testing to reduce the number of patients lost to follow-up.
Many jurisdictions have regulations requiring laboratories to report all positive results of hepatitis C markers to the HD, and some also receive negative laboratory results (anti-HCV and/or HCV RNA). Liver function tests (ALTs and total bilirubin results) can provide information on acute infection status; if testing is conducted as part of a pregnancy panel, pregnancy test results can identify HCV-infected pregnant people. To obtain data that will enable HCV infection status to be determined and follow-up care received, jurisdictions should have all positive laboratory results indicative of HCV infection reportable (i.e., anti-HCV, HCV RNA, HCV genotype, and any other tests indicating the presence of HCV). Section 4.4 (Recommended Reportable Laboratory Markers) provides information on what might be made reportable in different jurisdictions and the rationale for collecting these results. Table 4-1 describes the interpretations of hepatitis C laboratory results and actions that should be taken by anyone advising confirmed HCV-positive patients about necessary next steps (97). Anti-HCV indeterminate/borderline results are not interpretable and should be retested according to the Instructions for Use provided with the test assay.
Table 4-1. Interpretation of hepatitis C laboratory results
| Test Outcome* | Interpretation† | Further Actions |
|---|---|---|
| Nonreactive hepatitis C virus (HCV) antibody | No HCV antibody detected | No further action required in most cases. Though this would not be considered a case, some jurisdictions do require reporting (especially among children <36 months of age). There might be some instances where further testing is recommended.‡ |
| Reactive HCV antibody§ | Presumptive hepatitis C | A reactive result is consistent with current HCV infection, past HCV infection that has resolved, or biologic false positivity for HCV antibody. Recommend testing for HCV RNA to identify current infection. |
Reactive HCV antibody AND
|
Current hepatitis C | Provide patient with appropriate counseling and linkage to care. |
Reactive HCV antibody AND
|
Cleared hepatitis C | Result might be consistent with natural clearance or successful treatment or with a false-positive HCV antibody result. No further action required in most cases. Further testing may be recommended in some instances.¶ |
Table modified from https://www.cdc.gov/mmwr/pdf/wk/mm62e0507a2.pdf [PDF – 4 pages].
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate. No HCV antigen tests have been approved by the US Food and Drug Administration (FDA). When an FDA-approved test becomes available, it will be acceptable laboratory criteria, equivalent to HCV RNA testing. For surveillance purposes, the reporting of positive genotype test results should be considered equivalent to HCV RNA detection, as RNA is required for this test. However, a genotype test in which the genotype cannot be determined is not the same as a “not detected” HCV RNA result.
†Ingestion of high levels of biotin can significantly interfere with certain commonly used biotinylated immunoassays and cause false-positive or false-negative laboratory test results. Currently, the FDA is investigating thresholds associated with false-positive and false-negative tests. Reference: https://www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication.
‡Further testing might be recommended if a recent HCV exposure is suspected in the past 6 months (or longer in people who are immunocompromised) or if there is concern regarding the handling or storage of the specimen. If recent exposure is suspected, test for the presence of virus using either a NAT for HCV RNA or a test for HCV antigen (if available). If HCV RNA testing is not feasible, conduct follow-up testing for HCV antibody to demonstrate test conversion.
§If the HCV RNA result is indeterminate, consider provider follow-up to discuss interpretation of result and re-testing strategy.
¶Further testing might be recommended if a recent HCV exposure is suspected in the past 6 months, or if there is concern regarding the handling or storage of the specimen. If distinction between true positivity and biologic false positivity for HCV antibody is desired and the sample is repeatedly reactive, testing with an alternative HCV antibody assay may be useful. In certain situations (e.g., suspected HCV infection within the past 6 months, clinical evidence of HCV infection, and questionable specimen integrity), follow up with another HCV RNA test and appropriate counseling.
Downloads of this table: PDF | PPT
Recommended Reportable Laboratory Markers
The following laboratory markers are recommended for reporting to public health to aid in case ascertainment, case classification, and monitoring cure continua for hepatitis C:
- Anti-HCV (all positive results, negative results for children <36 months of age);
- HCV RNA (positive/detectable and negative/undetectable results), including quantitative, qualitative, and genotype testing;
- HCV antigen (positive, negative, and indeterminate results) when and if a test is approved by FDA; and
- If any of the above positive results are reported, also report the following:
- Pregnancy status,
- Concurrent ALT and total bilirubin results,
- Other hepatitis serological results (e.g., hepatitis A, hepatitis B, and/or hepatitis E).
Jurisdictions are strongly encouraged to incorporate the reporting of negative/undetectable HCV RNA test results into their surveillance regulations and systems to support improved understanding of their local epidemic. Such reporting may increase awareness regarding
- acute infections (new and re-infections) and cleared (resolved and cured) infections,
- completeness of testing, and
- availability of reflex testing in accordance with complete HCV testing recommendations dated July 2023 (95).
Jurisdictions might also wish to receive negative anti-HCV results to assist in identifying cases of test conversion and examine trends in screening; however, they must be mindful of their ability to process and store high volumes of data. Further, caution must be taken in the collection and use of these results, as people with non-reactive anti-HCV tests do not have a reportable condition. Jurisdictions must have legal authorization for receipt of these data.
In 2019, of 43 state, territorial, and major city HDs participating in the National Alliance of State and Territorial AIDS Directors (NASTAD) viral hepatitis surveillance and prevention capacity assessment, 17 (40%) received negative HCV RNA test results, and nine (21%) received negative anti-HCV test results. An additional 12 (28%) jurisdictions indicated that they received negative HCV RNA test results, and 10 (23%) indicated that they received negative anti-HCV test results, but either did not mandate negative hepatitis C laboratory reporting in their jurisdiction or were in the process of changing local laws or regulations to require reporting of negative hepatitis C laboratory results. Some jurisdictions have changed policy to allow reporting of negative HCV test results but have not yet modified their surveillance system to receive and process these negative test results because of limited resources and competing priorities.
Case Reporting and National Notification
Cases of acute, chronic, and perinatal hepatitis C and hepatitis C during pregnancy should be reported to HDs as specified by state, territorial, or local regulations. Acute, chronic, and perinatal hepatitis C are nationally notifiable conditions (5). Hepatitis C cases are identified using an event code corresponding to the hepatitis C condition (Table 1-2). Data are sent weekly or more frequently, depending on the infrastructure of the jurisdiction sending the data. Cases might be re-classified or removed as needed after the initial transmission to CDC, as long as the changes occur before surveillance data are finalized each year.
Surveillance of Acute and Chronic Hepatitis C
Uses of Surveillance Data
Surveillance Case Definitions
Case Ascertainment
Case Investigation
Case Reporting and National Notification
Surveillance Activities for Chronic Hepatitis C
Considerations for Hepatitis C Cases who were Transplant Recipients
Monitoring Infection Trends and Disease Outcomes Using a Person-Level Database and Supplemental Data Sources
Background
New cases of acute hepatitis C have increased rapidly in the United States since 2010, most being associated with IDU. The highest incidence of acute hepatitis C is typically found among people in younger age groups. For hepatitis C surveillance statistics for the United States, visit the CDC Viral Hepatitis Surveillance website (17).
Most people with chronic hepatitis C are asymptomatic (86, 98, 99); however, approximately 10%–20% of people living with chronic hepatitis C who have persistent liver inflammation will develop cirrhosis over the course of 20 years, and people with cirrhosis are at risk for developing liver cancer and other serious consequences (100). Those with hepatitis C who do not develop liver-related complications can still suffer from extrahepatic manifestations of chronic hepatitis C (e.g., severe fatigue, certain types of renal diseases, and certain autoimmune diseases) (101-104). Additionally, those living with chronic hepatitis C can continue to transmit the infection to others, and antibodies to HCV are not protective against reinfection (105).
Improving hepatitis C surveillance is an important component of national, state, and local strategies for eliminating hepatitis C as a public health problem. In addition to the general goals of viral hepatitis surveillance (Section 1.2), the overall goals of chronic hepatitis C surveillance are to measure and characterize the burden of infection and disease, and if feasible, create person-level systems/registries. Person-level data enable classification of those infected along the care continuum, from screening and diagnosis to linkage to care, treatment, and cure, helping jurisdictions inform and evaluate the impact of hepatitis C elimination activities. Goals specific to chronic hepatitis C surveillance include:
-
- monitoring trends in the prevalence of chronic infection;
- identifying cases for further investigation to better describe the epidemiology, including characterizing behaviors or exposures related to infection and identifying health disparities;
- detecting and responding to clusters and/or outbreaks (many acute infections have no clinical signs and will be classified as chronic cases);
- identifying infected people who require linkage to care and harm reduction resources, including through matches with other surveillance registries for HIV, cancer, and hepatitis B; and
- cross-referencing person-level systems/registries with vital statistics data to assess the burden of hepatitis C-associated deaths and perinatally-acquired hepatitis C.
Uses of Surveillance Data
Acute and chronic hepatitis C surveillance data can be used to inform and improve public health interventions in the following ways:
- Monitoring trends in disease incidence and determining risk behaviors or exposures. Acute hepatitis C surveillance data should be analyzed at regular intervals by person, place, and time to monitor disease incidence. Keep in mind that place might not simply be the provided address of residence, as location of occurrence of risk behaviors might not correspond to the reported address. Risk behavior or exposure information should be analyzed to monitor disease transmission patterns and to identify groups at higher risk for infection for whom prevention efforts should be targeted. Prevention efforts include vaccination for hepatitis A and hepatitis B, expanded access to PrEP to prevent HIV transmission, increased testing for bloodborne diseases, improved access to harm reduction services, and increased access to hepatitis C treatment and SUD treatment including MOUD.
- Identifying outbreaks. An outbreak is defined as the occurrence of more cases of disease than expected in a given area or among a specific group of people over a particular time period. Detailed guidance on viral hepatitis outbreaks, including examples of hepatitis C outbreaks, can be found on the CDC DVH Viral Hepatitis Outbreaks website (106).
- Assessing missed opportunities for prevention. Surveillance data can be used to provide information on cases occurring among adults at higher risk for infection to identify opportunities for intervention and prevention. Tools (e.g., a cure continuum) can be developed to identify performance measures for prevention of transmission and for access to care and treatment.
- Identifying needs for education. Surveillance data can help identify trends in risk or testing patterns and allow for education efforts (targeted to both health care providers and the general public) about transmission, long term consequences of chronic infection, and availability of treatment.
- Tracking cases of chronic hepatitis C. Surveillance systems and databases that track chronic hepatitis C cases can aid in monitoring trends in the prevalence of chronic infection.
- Understanding the burden of hepatitis C in the community. Person-based longitudinal databases can help describe the hepatitis C cure continuum in jurisdictions, including
- identifying people with positive anti-HCV results who have no HCV RNA result and need HCV RNA diagnostic testing (probable cases),
- identifying health-related disparities where hepatitis C affects specific sub-populations,
- monitoring for perinatal transmission (being born to an HCV-positive gestational parent) to ensure appropriate testing and identification of confirmed infections,
- monitoring movement of cases in or out of the jurisdiction, and
- tracking the occurrence of related adverse health outcomes.
- Identifying chronic HCV-infected people who need linkage to care. Surveillance data can be used to identify and follow-up on chronic hepatitis C cases (especially those who have been recently diagnosed), link them to appropriate medical care and harm reduction services, and ensure contacts are referred to testing and care, as appropriate.
Surveillance Case Definitions
Table 4-2 specifies the surveillance case definitions for acute and chronic hepatitis C, adopted by CSTE and CDC in 2020 (14, 107, 108). See Appendix C for classification scenarios of cases of acute and chronic hepatitis C.
Table 4-2. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definitions for acute and chronic hepatitis C, 2020
| Criteria Type | Criteria |
|---|---|
| Age |
|
| Clinical |
|
| Confirmatory Laboratory |
|
| Presumptive Laboratory |
|
| Anti-HCV Test Conversion |
|
| HCV Detection Test Conversion‡ |
|
| Case Status | Classification |
| Confirmed Acute‡ |
|
| Probable Acute‡ |
|
| Confirmed Chronic‡ |
|
| Probable Chronic‡ |
|
*At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
†The presence of a negative HCV detection test result, in the absence of criteria that would allow for confirmation, indicates that the case should not be classified as probable and should not be reported to CDC.
‡Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate.
§Timing of these tests may change as standard of care for HCV treatment evolves. Some jurisdictions are creating a local condition specific for reinfection as opposed to creating a new acute condition to maintain deduplication.
Downloads of this table: PDF | PPT
People who test positive for anti-HCV and have a negative HCV detection test (with no known prior positive HCV detection test) received before the data closes for that reporting year should be classified as “not a case” and should not be notified to CDC. People who test positive for anti-HCV and HCV RNA are still considered confirmed cases even if they later clear their infection (i.e., negative for HCV RNA). The critical differentiation for the case definition between acute cases and chronic cases is the presence of clinical criteria (i.e., jaundice or elevated total bilirubin or elevated ALT) in the absence of a more likely diagnosis.
To be classified as a probable acute case, provider reports of jaundice or laboratory reports of elevated total bilirubin or ALT must have been received within the reporting year prior to data close-out. Additionally, if HCV RNA is detectable and anti-HCV is undetectable on the same specimen, this could indicate laboratory evidence of early acute hepatitis C when anti-HCV testing was performed during the window period. See Section 5.1.3. on Laboratory Results Indicating Early Acute Hepatitis C for more information.
Cases are classified using the CDC/CSTE case definition at the time the case is reported. A person’s case status might change throughout the year as more test results are reported. In addition, a confirmed or probable acute case might be classified as a new confirmed chronic case in a subsequent reporting year, if a positive HCV detection test is reported >12 months after the collection date of the first positive test indicating acute infection (14).
Although jurisdictions have varying capability to track reinfection, evidence of reinfection might include someone who was previously a confirmed hepatitis C case who then had at least two sequential negative HCV detection tests at least 12 weeks apart, followed by a positive HCV detection test. Assessment for SVR ≥12 weeks after the completion of treatment is the same for treatment-naïve patients with and without cirrhosis according to the simplified hepatitis C treatment guidance (109). Other evidence of reinfection should be considered, including a report of a new genotype from a case who had previously cleared an infection from a different genotype (14). Jurisdictions are encouraged to take measures to ensure that cases of hepatitis C treatment failure are not classified as new cases of hepatitis C (14).
One option for tracking and investigating reinfections is to create a local condition called “possible hepatitis C virus reinfection or possible hepatitis C virus treatment failure” as opposed to creating a new acute condition to maintain a deduplicated registry. Jurisdictions tracking reinfections should consider requesting medical records and collecting data on prior treatment completion (when relevant and possible to document), treatment failure, and the known time frame for reinfection in order to determine true reinfections from possible treatment failures (14).
HCV RNA results might be reported as below the lower limit of quantification (e.g., <15 IU/mL) yet simultaneously indicate that HCV RNA was detected. HCV RNA results that are above the limit of detection but below the lower limit of quantification should be considered “detectable” or “positive” for surveillance classification purposes (95).
Case Ascertainment
The primary method for ascertaining suspected cases is by investigating reports from clinical laboratories, health care facilities, and health care providers suggestive of hepatitis C. Rules or regulations requiring facilities and providers to report hepatitis C to public health agencies vary by jurisdiction. See Section 1.6 and Section 4.4 for information on the recommended reporting requirements for hepatitis C.
Laboratory Reporting
Laboratory reporting of HCV infection is required in all states for which acute and chronic hepatitis C is reportable. While case-defining infection markers (e.g., positive HCV RNA tests) are reportable in most jurisdictions, regulations vary regarding which positive indicators within the panel must be reported. It is recommended that jurisdictions require reporting of all negative/undetectable HCV RNA results (to monitor jurisdictional cascade of care), plus negative anti-HCV results in children <36 months of age (for perinatal hepatitis C surveillance). Complete reporting of all tests in a hepatitis panel, to include negative hepatitis C laboratory results, allows public health officials to more accurately interpret results. However, this also requires more sophistication in information systems to efficiently send, process, and utilize the information received.
Health Care Facility and Provider Reporting
Many states require health care facilities and providers to report hepatitis C diagnoses.
Additional sources of information include medical records, hospital discharge databases, death certificates, and birth certificates. Section 5.4 provides more information on these data sources. Figure 4-2 illustrates a potential approach for acute and chronic hepatitis C case ascertainment and classification. Specific procedures might vary by jurisdiction, but should generally follow the scheme below, in accordance with the CDC/CSTE Position Statement for the 2020 acute and chronic hepatitis C case definitions (14, 107, 108).
Cases among children 2–36 months of age should be classified under the Perinatal Hepatitis C Position Statement (17-ID-08) case definition unless the exposure mode is not perinatal (e.g., health care-associated). See Section 4.7.4 for case ascertainment guidance of perinatal hepatitis C cases.
Figure 4-2. Process for acute and chronic hepatitis C case ascertainment and classification
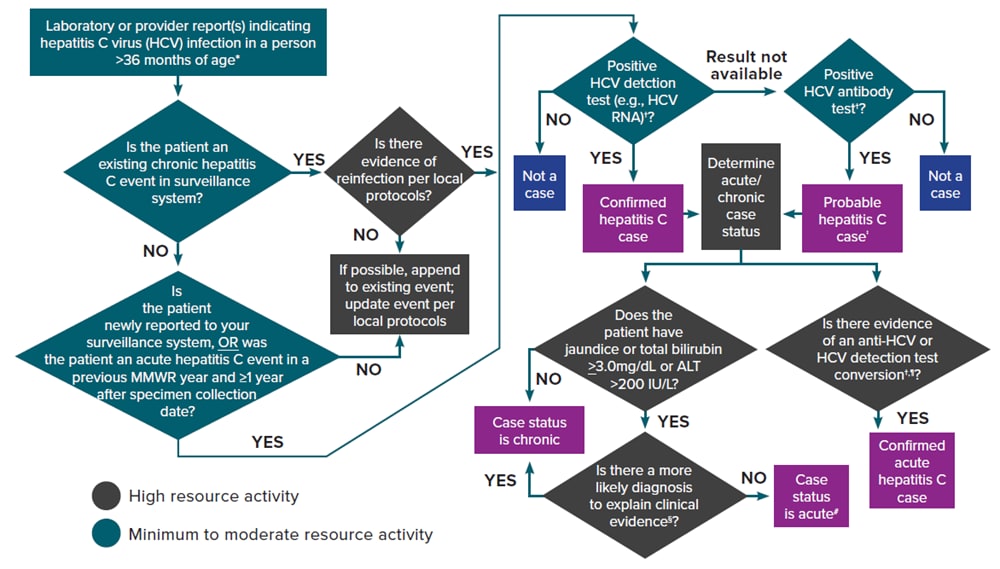
*A child <36 months of age whose mode of exposure is not perinatal (e.g., health care-acquired) should be classified under the 2020 acute or chronic hepatitis C case definition. A child 2–36 months of age whose mode of exposure is perinatal should be classified under the 2018 perinatal hepatitis C case definition.
†Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate. HCV detection testing includes nucleic acid testing for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
‡May re-classify as confirmed if a positive HCV detection test is later received before the National Notifiable Diseases Surveillance System (NNDSS) close-out date for national notification purposes. Jurisdictions with a longitudinal system can update probable cases to confirmed within their system at any time regardless of the NNDSS close-out date.
§May include evidence of acute liver injury from infectious, autoimmune, metabolic, drug or toxin exposure, neoplastic, circulatory or thromboembolic, or idiopathic causes.
¶A documented negative HCV antibody followed within 12 months by a positive HCV antibody test (anti-HCV test conversion) OR a documented negative HCV antibody OR negative HCV detection test (in someone without a prior diagnosis of HCV infection) followed within 12 months by a positive HCV detection test (HCV detection test conversion).
#A new, acute hepatitis C case is either an incident case that has not been previously reported or a case among someone previously reported as having hepatitis C who has laboratory evidence of reinfection (14). Some jurisdictions are creating a local condition specific for reinfection as opposed to creating a new acute condition to maintain a deduplicated registry.
Reference:
- Council of State and Territorial Epidemiologists. Position statement 19-ID-06: Revision of the case definition for hepatitis C. Available at: https://cdn.ymaws.com/www.cste.org/resource/resmgr/2019ps/final/19-ID-06_HepatitisC_final_7..pdf [PDF – 11 pages]. Accessed on January 16, 2020.
Downloads of this figure: PDF | PPT
Case Investigation
The original case report may be sufficient to classify a case of hepatitis C as being acute or chronic. Resource limitations may not allow all chronic cases to be investigated in the same way as acute cases. Additional investigation may be necessary depending on the priority level of the case. The level of investigation will depend on the situation, the objectives, and the available resources. Below is a description of the type of information that should be collected during case investigations.
Information from the Laboratory
Newly reported positive anti-HCV and HCV detection laboratory results should be reported to the HD. Concurrent ALT and total bilirubin results reported with positive hepatitis C laboratory results can be helpful in identifying cases that might be acute.
Information from the Provider or Medical Records
The following types of information might be available from the medical records:
- Demographic information. Includes name, date of birth, sex at birth, current gender, race, ethnicity, and residential address (including zip code).
- Clinical features. Includes reason for testing, signs (jaundice) and symptoms (if available*), hospitalization status and date of death (if applicable), and whether an alternate diagnosis is suspected. HDs should inquire about the potential of past infection to confirm whether current clinical features are due to a newly acquired infection. The medical record might provide evidence of chronic liver disease.
*Includes fever, headache, malaise, anorexia, nausea, vomiting, diarrhea, and abdominal pain. - Pregnancy status. Pregnancy status should be checked for all people of childbearing age with childbearing potential. Children born to HCV-positive gestational parents should be tested for infection and classified according to the CDC/CSTE perinatal hepatitis C case definition.
- Diagnostic test results. If additional laboratory testing (e.g., ALT levels, total bilirubin levels, and results from a hepatitis panel) is needed to classify the case.
- Risk behaviors or exposures. Includes history of IDU, sexual contact resulting in exposure to blood (e.g., anal intercourse), experience of homelessness, recent medical procedures, hemodialysis, incarceration, and residence in a long-term care facility.
Information from the Patient
Unless the source of infection is known (e.g., transplantation of an organ from an HCV-positive donor into an HCV-negative recipient), all patients with acute hepatitis C should be contacted for an interview using the jurisdiction-specific acute hepatitis C case investigation form. If resources are limited, at a minimum, all patients who are classified as “confirmed” per the CDC/CSTE case definition and those flagged as having public health importance (Section 4.2) should be interviewed. Decisions to contact the patient are often jurisdiction-specific and depend on the resources available. In many situations, patient contact might be reserved for those cases deemed highest priority for preventing further transmission or for referral for additional care and treatment, as needed. The patient interview should ideally include the following components:
- Risk behaviors or exposures. To identify a potential source or risk behavior or exposure(s) for infection during the 2 weeks to 6 months prior to illness onset. For chronic cases, if it is determined that the person has current risk behaviors or exposures for ongoing transmission or was identified as part of a cluster of cases, additional information might be prioritized.
- Education and referral for follow-up. People with newly diagnosed acute and chronic hepatitis C should be advised on how to prevent transmission to others. HDs should assess whether the patient requires education, provider referral for treatment, and other medical and public health follow-up services (e.g., hepatitis A and hepatitis B vaccination, PrEP to prevent HIV transmission, MOUD, SSPs, and/or harm reduction services), as appropriate.
- Identification of contacts requiring testing and vaccination. If resources allow, identify contacts and coordinate testing, counseling, linkage to care, and hepatitis A and hepatitis B vaccination in accordance with existing recommendations by ACIP. Identification of contacts might require further work-up to identify HCV infection networks that could potentially result in an outbreak.
Special Considerations when Investigating Certain Populations or Settings at Risk for Rapid Disease Transmission
Considerations when investigating hepatitis C cases among certain populations at risk for rapid transmission are provided in Section 1.10.
Case Investigation Prioritization
The automated collection of hepatitis C laboratory results will, in many jurisdictions, lead to a high volume of reporting. Even with automated reporting, many HDs lack the resources needed to conduct investigations for all acute cases. Jurisdictions might consider the following when prioritizing cases for follow-up:
- Require providers to report clinically identified acute infections directly to the HD
- If resources allow, automate the collection of ALT and total bilirubin results through ELR or EMR reporting, and prioritize data collection to confirm those cases with abnormal results
- Conduct semi-automated/preliminary collection of risk data combined with more targeted follow-up on cases WITHOUT anticipated risk history
- Target efforts to demographic groups that might be at higher risk of acquiring or transmitting infection
- Pregnant people
- New infections reported in elderly patients (e.g., >70 years of age)
- People <40 years of age that might represent emerging risks
- People infected with HIV*
- Target efforts based on specific settings within a jurisdiction
- SSPs or substance use disorder treatment facilities
- Correctional facilities
- Retirement/nursing facilities
- Homeless services providers
- Areas where known risk behaviors are occurring, or rates of newly reported infections are increasing
- Implement efficient data collection
- Test in public health clinics
- Supplement case-surveillance data with data sources to provide information about higher risk populations and the evolving epidemiology of acute infections
- SAMHSA/state drug use, overdose, and EMS data
- HIV incidence data to identify coinfection*
- Ongoing outbreak and cluster investigations, if applicable
- Hospital discharge data
Considerations for Conducting a Chronic Hepatitis C Case Investigation
Conducting an investigation for a chronic hepatitis C case can involve the following considerations:
- Check the jurisdiction’s hepatitis C registry/surveillance system to ensure the case is newly reported and not previously documented.
- Review the information in the initial report to determine if the case falls within a group prioritized for investigation, such as those outlined in Section 4.2. At a minimum, pregnancy status should be checked for all people with chronic hepatitis C who are of childbearing age with childbearing potential; reports of HCV-infected pregnant people should be shared with the staff member responsible for perinatal hepatitis C case management.
- When possible, contact the health care provider and/or review medical records to obtain additional information to help prioritize which cases should receive a patient interview.
- For patients who are interviewed, collect relevant demographic and risk history information using the jurisdiction-specific case report form.
- Investigate likely health care exposures according to the jurisdiction’s procedures, ideally in collaboration with the health care-associated infection team.
- Provide patient education about ways to avoid the spread of infection to others and ways to avoid further harm to the liver.
- Educate people who have had direct exposure to the patient’s blood about HCV transmission and provide hepatitis C testing if they are not known to be infected.
- If the case is in a child, screen the parents and household members for evidence of infection.
- If resources allow, contact the provider and/or refer the patient to a patient navigator to ensure the patient is in care and receives treatment.
Case Reporting and National Notification
Cases of acute and chronic hepatitis C are nationally notifiable to CDC using a condition-specific event code (Table 1-2). Acute and chronic cases can be re-classified, removed, or changed after the initial transmission to CDC as long as revisions are made before surveillance data are finalized each year. A case initially transmitted to NNDSS as probable might later be reclassified as “confirmed” or “not a case.” A confirmed acute case may be classified as a confirmed chronic case if a positive HCV detection test is reported one year or longer after acute case onset. All confirmed and probable cases are recommended to be transmitted to NNDSS for inclusion in CDC case counts in print criteria.
Surveillance Activities for Chronic Hepatitis C
Due to varying levels of resources, jurisdictions might be at different stages of implementing surveillance activities for chronic hepatitis C. The following section provides best practice models for core and enhanced surveillance activities for consideration by jurisdictions. Enhanced surveillance activities should be identified based on local priorities.
Best Practice Models for Core and Enhanced Chronic Hepatitis C Surveillance
Core Surveillance
Case Ascertainment and Reporting
- Create an electronic system for systematically collecting and storing hepatitis C test results and other case data (e.g., demographic, risk, and clinical information) longitudinally for unique (de-duplicated) persons.
- Establish a method to receive hepatitis C laboratory data and enter into the hepatitis C system/registry, preferably through an automated ELR system. ELR is the most efficient way to receive these data, especially if the ELR system can automatically enter the hepatitis C records into the surveillance system.
- Jurisdictions with an existing ELR system for other conditions can incorporate hepatitis C testing.
- If ELR is not possible, work with high volume testers to receive data another way (e.g., periodic flat files).
- Determine whether hepatitis C cases will be updated within the surveillance system/registry as new laboratory reports are received (e.g., case status, patient address, and pregnancy information) or whether only laboratory reports received at the time the case investigation is created will be considered.
- Implement a process to extract data from hepatitis C system/registry, classify case investigations, and transmit to CDC according to procedures for the National Notifiable Diseases Surveillance System.
Investigations
- Document local procedures for case investigations, including defining priority populations (see Section 4.2).
- Conduct case investigations for priority populations where feasible (see Section 4.2). Surveillance activities include but are not limited to reviewing EMRs, communicating with providers and/or health care facilities via phone or facsimile, and interviewing patients to collect demographic, risk, and clinical information and other data deemed necessary.
- Establish a protocol for identifying and investigating health care-associated infections*. Depending on the structure of the health department, this might be conducted separately from hepatitis C surveillance with assistance from health care-association infections staff. Use CDC’s health care-associated infection toolkit as a resource: https://www.cdc.gov/hai/outbreaks/outbreaktoolkit.html.
- Establish a protocol for identifying and investigating other unique exposures, including clusters and/or outbreaks of hepatitis C*.
*Some newly reported cases meeting the chronic hepatitis C case definition may reflect asymptomatic acute infections.
Quality Assurance
- Establish a process for data cleaning and standardizing laboratory reports.
- Assess case investigations and laboratory reports for completeness and accuracy.
- Identify and review potential duplicate laboratory reports, patients, and/or case investigations.
Analyses
- Create an annual report, situational analysis, or other data product that can be widely shared with providers, advocates, stakeholders, and other public health professionals.
Policy
- Research existing health code/policy related to hepatitis C reporting and the process for changing such policies (if necessary).
- Identify who should report hepatitis C cases (e.g., health care providers, health care facilities, and/or laboratories).
- Determine what should be reportable. At a minimum, positive anti-HCV, positive NAT for HCV RNA (including qualitative, quantitative, or genotype testing), or a positive test indicating presence of HCV antigen should be reportable. If possible, pregnancy status and concurrent ALT and total bilirubin results should be reported with positive hepatitis C laboratory results, and negative HCV detection test results should also be reported. Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate. At present no HCV antigen tests are approved by the FDA. These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Data Sharing
- Research how to obtain access to supplemental sources of data (e.g., data from vital statistics, cancer registry, and HIV registry) to match to the hepatitis C registry.
Enhanced Surveillance
Case Ascertainment and Reporting
- Implement a process for updating cases in the system/registry with potential treatment/cure data to track patients along the hepatitis C cure continuum.
- Use additional data sources to identify cases not previously reported through other means (e.g., pharmacy claims for hepatitis C treatment, hospitalization data, payer records, vital records, and chart review).
- Use additional data sources to supplement data in the system/registry (see Section 5.4). The following are examples of ways to use such data sources.
- Conduct vital statistics death registry matches to update vital status and death date.
- Conduct vital statistics birth registry matches to update pregnancy information and to link gestational parent-infant pairs within the surveillance system.
- Conduct data linkage matches to other disease registries (e.g., HIV and cancer) to find missing information (e.g., race/ethnicity) and to assess and address coinfection and comorbidities.
Investigations
- Conduct chronic hepatitis C case investigations for additional priority populations (see Section 4.2).
- Draft an outbreak response plan that includes jurisdictional actions for hepatitis C clusters and/or outbreaks.
- Establish methods for identifying reinfections (confirm the case was previously treated and cured) to establish reinfection rates and target prevention efforts.
- If personnel and other resources allow, consider in-depth investigation of a random sample of chronic cases to evaluate demographic variables, reason for testing, access and barriers to prevention and treatment services, and other questions of importance for viral hepatitis elimination activities in the jurisdiction. Personnel with expertise in study design, data collection, and analytic skills should develop and oversee these types of in-depth investigations.
- Assure linkage to care, treatment, and harm reduction services for priority populations where resources allow.
- Use detection software (e.g., SaTScan) to identify potential hepatitis C clusters and/or outbreaks*.
- Use molecular sequencing (Global Hepatitis Outbreak Surveillance Technology [GHOST]) to establish hepatitis C virus (HCV) transmission linkage for cluster and/or outbreak investigations*.
*Some newly reported cases meeting the chronic hepatitis C case definition may reflect asymptomatic acute infections.
Quality Assurance
- Establish quality assurance processes for chronic hepatitis C case data.
- Implement quality improvement measures to ensure completeness and accuracy of case investigations and interpretation of laboratory reports.
- Establish systems to identify and address decreases in hepatitis C laboratory reporting by test type volume and laboratory that might represent coding or transmission issues.
- Establish systems to identify and address deficiencies in provider reporting (e.g., incomplete or missing hepatitis C reports) that might represent coding or transmission issues.
Analyses
- Create provider-level indicators (e.g., complete reporting, complete diagnostic testing, linkage to care, and treatment initiation) to work with health plans and health care providers to improve these outcomes.
- Use data linkage matches to other disease databases/registries (e.g., HIV) for analysis of co-infections and implementation and evaluation of data-to-care interventions.
- Use vital statistics birth registry matches for analysis of infants born to HCV-positive gestational parents.
- Use death registry matches to describe hepatitis C-associated mortality.
- Describe trends and disparities in liver cancer incidence and mortality via linkage with jurisdictional cancer registry.
- Identify methods for establishing surveillance-based hepatitis C prevalence estimates.
- Create hepatitis C cure continua, including determining and validating surveillance-based definitions for hepatitis C treatment and cure.
- Describe trends and disparities along the hepatitis C cure continuum (e.g., disparities in screening, viremia, linkage to care, treatment initiation, cure, and reinfection).
Policy
- Use hepatitis C surveillance data (e.g., assessing the proportion of people with anti-HCV positive results and no known HCV RNA result) to support evidence-based health code changes related to testing and reporting (e.g., mandatory reflex HCV RNA testing and reporting of negative HCV detection test results).
- Use surveillance data to assess unmet needs for prevention and harm reduction services, and to support evidence-based health code changes related to expanding access to syringe services programs and other harm reduction services for populations affected by hepatitis C.
- Use analysis of trends and disparities to guide resource allocation and inform public health action, prioritizing those communities most disproportionately affected.
Data Sharing
- Obtain access to supplemental data sources wherever possible and incorporate their usage into routine practices. See Section 5.4 for a description of optional data sources.
Considerations for Hepatitis C Cases who were Transplant Recipients
With the availability of curative treatment for HCV infection, an increasing number of transplant recipients are receiving organs from anti-HCV and HCV-RNA positive donors (110). This can result in transmission of hepatitis C to the recipient, which is then treated with DAA agents (111). In some jurisdictions, these expected donor-derived HCV transmissions might represent a significant proportion of new acute HCV infections; therefore, jurisdictions are encouraged to reach out to transplant facilities and discuss public health reporting of expected donor-derived HCV infections.
A listing of transplant facilities in the United States, including facility location and phone number, can be found on the OPTN website (71). As these patients are already linked to testing and treatment, the infections should be notified to CDC as new acute cases. However, the jurisdiction need not investigate beyond indicating that the infection was donor-derived.
Jurisdictions might also get reports of unexpected donor-derived HCV infection. Unexpected infection occurs rarely when both donor and recipient are HCV RNA negative pre-transplant, usually in situations where the donor was infected (e.g., actively injecting drugs) shortly before demise (66). When a suspect donor-derived acute hepatitis C case is identified, the transplant center is required to report the infection to OPTN’s DTAC that might request assistance from the CDC Office of Blood, Organ and Other Tissues Safety. If CDC accepts the investigation, CDC DVH epidemiologists will work with the laboratory to conduct any testing and reach out to the jurisdiction when that part of the investigation is complete.
Typically, there are two outstanding questions that only the public health jurisdiction can answer: 1) Did the recipient have any behavioral or other risks for hepatitis C and 2) Does the jurisdiction have any ongoing investigations of health care-associated hepatitis C that might be related to this investigation?
Case classification in patients with a documented transplant should consider reports of laboratory test results prior to and post-transplant and potential health care exposures, if suspected. Table 4-3 outlines considerations for hepatitis C cases who were organ transplant recipients.
Table 4-3. Considerations for hepatitis C cases who were organ (or tissue) transplant recipients*
| Organ Recipient Pre-transplant
Laboratory Result† |
Organ Recipient Post-transplant
Laboratory Result† |
Case Classification |
|---|---|---|
| Positive HCV antibody (anti-HCV) AND positive HCV detection test‡ | Positive HCV detection test‡ | Should not be considered a new case due to organ transplant, but rather an infection documented prior to transplant§. To determine whether this case should be considered newly reported, follow Figure 4-2. |
| Positive anti-HCV with evidence of cure according to AASLD/IDSA hepatitis C treatment guidelines | Positive HCV detection test‡ | Should be classified as an acute infection due to reinfection according to the CDC/CSTE case definition and investigated with three major hypotheses in mind:
CDC’s Division of Viral Hepatitis might already have been notified about the investigation and is available for consultation. |
| Negative anti-HCV AND negative HCV detection test‡ | Positive HCV detection test‡ | Should be classified as an acute infection according to the CDC/CSTE case definition and investigated to identify the source of transmission with 3 major hypotheses in mind:
CDC’s Division of Viral Hepatitis might already have been notified about the investigation and is available for consultation. |
| No prior HCV laboratory results¶ |
*It is recommended that donors undergo anti-HCV and HCV RNA testing prior to organ procurement (63). If donors are negative for HCV RNA, transmission is considered “unexpected.” Transmission has occurred from donors who were infected/re-infected shortly before death; in this scenario, transmission to the recipient occurs during the “window period” (62).
†Because of the large number of tests performed on recipients, irreproducible positive results are sometimes reported. Investigators should review all results in context. CDC’s Division of Viral Hepatitis is available for consultation.
‡The 2020 Public Health Service (PHS) guidelines recommend testing all organ recipients for anti-HCV and HCV RNA pre-transplant and for HCV RNA at 4–5 weeks post-transplant (63).
§If the pre-transplant genotype differs from that observed post-transplant, consider investigating as if the infection is newly acquired.
¶All recipients should be tested pre-transplant for anti-HCV and HCV RNA. If the recipient has not been tested appropriately pre-transplant, consider contacting the transplant center to promote awareness of the 2020 PHS guidelines.
References:
Council of State and Territorial Epidemiologists. Position statement 19-ID-06: Revision of the case definition for hepatitis C. Available at: https://cdn.ymaws.com/www.cste.org/resource/resmgr/2019ps/final/19-ID-06_HepatitisC_final_7..pdf [PDF – 11 pages]. Accessed on January 16, 2020.
American Association for the Study of Liver Diseases/Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at https://www.hcvguidelines.org/ Accessed January 16, 2020.
Download of this table: PDF
Cases of viral hepatitis identified among living organ transplant donors and recipients should be submitted to NNDSS in a standardized way, when possible. The CDC case report forms used for NBS and HL7 transmission both include a reason for testing variable in the core section of the form. For state and territorial HDs transmitting data via NBS or HL7, under the “reason for testing” field, “blood/organ donor screening” should be selected for organ transplant donor cases and “other” should be selected for organ transplant recipient cases with a specification of “transplant recipient” in the free text under the “other reason for testing” field. For state and territorial HDs transmitting case data via NETSS, there is no field on the case report form to indicate that the case was an organ or tissue transplant donor or recipient.
Monitoring Infection Trends and Disease Outcomes Using a Person-Level Database and Supplemental Data Sources
A person-level surveillance database can support hepatitis C elimination efforts by allowing a jurisdiction to document a person’s hepatitis C laboratory testing history, including
- providing information on the number of people at each phase of the hepatitis C cure continuum to identify areas for improvement;
- tracking the number of unique persons living with hepatitis C longitudinally, which can inform more accurate estimates of incidence and prevalence;
- identifying pregnant people tested during prenatal care and perinatally exposed infants born to HCV-infected gestational parents;
- identifying and linking people living with hepatitis C to medical care;
- evaluating the impact of public health and clinical service; and
- matching with secondary data sources (e.g., Vital Statistics, Medicaid, cancer registry, and HIV jurisdictional registries).
Some of these patterns can only be determined for jurisdictions capable of capturing negative HCV RNA test results. Linking a person-level surveillance database to other data sources allows for longitudinal monitoring of disease outcomes and improves completeness of information in the surveillance system (72). Some jurisdictions have used their surveillance database to identify pregnancy status through routine matching with optional data sources. Supplemental data sources are helpful for understanding the burden of co-morbidities, such as infection with HBV and HIV, by providing cross-sectional data over time and can be used to inform interpretation of prevalence estimates. Section 5.4 describes supplemental data sources to consider.
Surveillance of Hepatitis C During Pregnancy and Perinatal Hepatitis C
Uses of Surveillance Data
Surveillance Case Definition
Case Ascertainment
Case Investigation
Case Management
Case Reporting and National Notification
Background
From 2009–2014, the prevalence of hepatitis C among pregnant people in the United States significantly increased by 89%, from 1.8 to 3.4 per 1,000 live births based on maternal HCV infection status reported on birth certificates from NVSS (110). Additionally, the proportion of infants born to HCV-infected gestational parents increased by 68% nationally from 2011 through 2014 (111).
Perinatal hepatitis C became nationally notifiable in 2018 (112). However, case identification of perinatal hepatitis C can be resource-intensive, and implementation of perinatal hepatitis C surveillance is not yet widespread. CDC prioritizes perinatal hepatitis C surveillance to prevent transmission and increase identification of hepatitis C in infants and children born to HCV-positive gestational parents.
To improve the prevention and identification of perinatal hepatitis C and facilitate clinical care for people who are pregnant or postpartum, CDC recommends hepatitis C screening during each pregnancy in settings where the HCV RNA prevalence is >0.1% or HCV RNA prevalence is unknown (76, 90). Because most settings are unlikely to have an HCV RNA prevalence as low as 0.1%, hepatitis C screening should be conducted in most settings.
The overall goals of surveillance of hepatitis C during pregnancy are to 1) determine whether pregnant people are currently infected with HCV (as indicated by the presence of HCV RNA) and 2) among HCV-positive people of childbearing age with childbearing potential, identify those who are currently pregnant or have recently delivered a live birth to identify perinatal HCV transmission.
The term “HCV-positive” is used when describing people who are HCV RNA-positive or who are anti-HCV-positive with no evidence of an HCV detection test being performed. Until the HCV detection status is known, surveillance should err on the side of inclusion for perinatal exposures and pregnant people. HCV-positive pregnant people should be linked to care for disease staging and treatment after pregnancy according to clinical recommendations, as DAAs can cure people of their HCV infection. Not only can curative hepatitis C therapy benefit people, but it can prevent HCV exposures during any future pregnancies.
Treatment during pregnancy is not currently recommended due to limited large-scale safety and efficacy data, though clinical trials are ongoing. Treatment during pregnancy may be considered on a patient-level basis after a physician-patient counsel about the possible risks and benefits (87). Children born to HCV-positive gestational parents should be linked to care for appropriate testing to identify potential perinatal HCV transmission.
As resources permit, surveillance of HCV infection during pregnancy should include monitoring each pregnancy as well as assessment of pregnancy status at multiple timepoints and not considering pregnancy to be a one-time event. When feasible, jurisdictions are encouraged to collaborate broadly with other partners addressing SUD in pregnant people to improve access to prenatal care and other services including post-partum treatment for hepatitis C.
The overall goals of perinatal hepatitis C surveillance are to ensure that infants born to HCV-positive gestational parents are identified, appropriately tested for hepatitis C, and linked to care. The additional goals of perinatal hepatitis C surveillance are to
- identify HCV RNA-positive gestational parents not previously identified during pregnancy and link them to care to prevent vertical HCV transmission during any future pregnancies;
- provide data to improve assessment of the burden of perinatal hepatitis C;
- evaluate health outcomes of infected infants;
- evaluate the overall effectiveness of perinatal hepatitis C programs;
- identify the appropriateness of HCV testing among children;
- educate clinicians and guardians on HCV transmission, clinical progression, and treatment; and
- measure the rate of progression to chronic hepatitis C, as determined by a positive HCV detection test result after 36 months of age.
Uses of Surveillance Data
Surveillance data on hepatitis C during pregnancy can be used to inform and improve public health interventions in the following ways:
- Identifying HCV-positive pregnant people to ensure linkage to hepatitis C-specific care. All HCV-positive people should be evaluated for care and treatment, when clinically indicated, by a medical provider.
- Identifying HCV-positive people who are pregnant or who have recently given birth to prioritize testing their infant for hepatitis C. Identification of an HCV-positive pregnant person during or after delivery allows for coordination of case management to ensure appropriate testing of their infant for perinatal HCV transmission. Such early identification of hepatitis C will result in fewer undiagnosed infections in the pediatric and young adult population and creates opportunities for linking infants to care so they can be evaluated for treatment with HCV DAAs at >3 years of age.
- Monitoring adherence to screening recommendations during pregnancy. To best monitor adherence to AASLD and CDC HCV screening recommendations among pregnant people, surveillance programs should ideally collect negative anti-HCV and negative HCV RNA results. Surveillance can help track changes in hepatitis C incidence among pregnant people or ensure implementation of quality measures to monitor adherence to screening recommendations.
- Monitoring trends in disease incidence and prevalence among people of childbearing age with childbearing potential. Knowing the incidence and prevalence of hepatitis C among people who are or have the potential to become pregnant is critical to the control, prevention, and ultimate elimination of HCV infection. Tracking this population with other chronic and acute hepatitis C surveillance data is adequate, but it should be assessed independently from surveillance of the general population.
Perinatal hepatitis C surveillance data can be used to inform and improve public health interventions in the following ways:
- Identifying children <36 months of age who test positive for anti-HCV and/or positive for HCV RNA. Early identification of children 18–36 months of age who test positive for anti-HCV and/or infants and children 2–36 months of age who test positive for HCV RNA will increase the number diagnosed with hepatitis C in the pediatric and young-adult population. Testing for anti-HCV at <18 months of age is not recommended as a positive result could be caused by trans-placental maternal anti-HCV. Curative DAA treatment can be provided to children as young as 3 years of age (92). Early identification is important to ensure access to early treatment.
- Monitoring trends in disease incidence among children 2–36 months of age. While perinatal hepatitis C surveillance data should be incorporated into data management systems for acute and chronic hepatitis C, monitoring incidence among children in this age range should close existing gaps in perinatal hepatitis C ascertainment.
- Monitoring and evaluating the effectiveness of perinatal hepatitis C programs. The following indicators can be used to monitor and evaluate the effectiveness of perinatal hepatitis C programs:
- The proportion of infants born to HCV-infected gestational parents who are
- Tested for hepatitis C and
- Tested for hepatitis C according to clinical guidelines.
- The proportion of perinatal hepatitis C cases who
- Receive additional testing,
- Test HCV RNA negative after 36 months of age,
- Test HCV RNA positive after 36 months of age (i.e., reported to CDC as chronic hepatitis C), and
- Receive medical evaluation and treatment, if appropriate.
- The proportion of infants born to HCV-infected gestational parents who are
Surveillance Case Definition
No CDC/CSTE surveillance case definition exists for HCV infection during pregnancy. Instead, these cases should be classified in accordance with the CDC/CSTE acute and chronic hepatitis C case definitions. See Section 4.6.3 for the acute and chronic hepatitis C case definitions.
Table 4-4 specifies the surveillance case definition for perinatal hepatitis C, CSTE and CDC in 2018 (114, 115). See Appendix C for classification scenarios of cases of perinatal hepatitis C.
Table 4-4. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for perinatal hepatitis C, 2018
| Criteria Type | Criteria |
|---|---|
| Demographic |
|
| Clinical |
|
| Laboratory* | Child <36 months of age with evidence of hepatitis C as shown by the following laboratory results:
|
| Epidemiologic Linkage |
|
| Case Status | Classification |
| Confirmed Perinatal* |
|
Downloads of this table: PDF | PPT
Test results prior to 2 months of age should not be used for classification. Cases among children in the specified age range that are known to have been exposed to HCV through a mechanism other than perinatal transmission should also be reported under the 2020 acute and chronic hepatitis C case definition.
HDs should be notified of children with a positive anti-HCV test performed at 18–36 months of age for whom no HCV detection test results have been reported, as this can represent perinatal HCV transmission. However, these cases should not be reported to CDC per the perinatal hepatitis C position statement case definition (115). HDs might consider classifying these children under a “suspected” or other non-notifiable case classification in their disease surveillance system for tracking and case management to confirm receipt of HCV RNA test results and identify siblings for whom HCV testing may be indicated.
Case Ascertainment
In 2020, CDC published the recommendation that all pregnant people be screened for HCV in settings where the HCV RNA prevalence is >0.1% or HCV RNA prevalence is unknown (93), increasing identification of both HCV infection during pregnancy and perinatal hepatitis C. All positive anti-HCV and positive HCV RNA tests should be reported to the responsible public health jurisdiction. See Section 4.4 for additional information on recommended reportable hepatitis C laboratory markers.
Pregnancy status should be considered routinely at multiple timepoints. Examples of timepoints for pregnancy status consideration include
- at the time of every new electronic laboratory report and
- when pregnancy status is provided to the jurisdiction by a laboratory or provider.
Regularly utilizing birth records that are matched to the HCV surveillance registry also can improve case ascertainment.
Determination of pregnancy status for all HCV-positive people who have the potential to become pregnant poses a significant challenge to local HDs, as this activity can potentially strain already limited public health resources. Jurisdictions should explore automated methods for receiving pregnancy status where possible, and if necessary, prioritize a subset of cases for follow-up of pregnancy status. The following are some examples of methods for determining pregnancy status that should be considered and incorporated when resources permit:
- Prioritizing follow-up of HCV-positive laboratory tests that were ordered by prenatal clinics or obstetrics and gynecology (OB/GYN) offices.
- Obtaining pregnancy status when investigations or follow-up is done on people with acute and/or chronic hepatitis C.
- Incorporating pregnancy status reporting within ELR (e.g., via HL7-based laboratory testing codes associated with ordering a prenatal screening panel), electronic medical record (EMR) reporting, and reporting from publicly funded testing sites. ELR messaging can be reviewed and automatically incorporated into data management systems when possible, utilizing analytical software coding to identify new pregnancy reports.
- Utilizing data matching with birth records to identify people who both recently gave birth and represent hepatitis C cases. These matches can be performed at various frequencies to improve timeliness of identification of an HCV-positive person who gave birth.
- Mandating the reporting of pregnancy for those known to be positive or newly tests positive for anti-HCV or HCV RNA, either from the laboratory or provider. Mechanisms for reporting include reporting via REDCap-based forms, electronic health records, facsimile, or electronic laboratory reporting of pregnancy status.
Perinatal hepatitis C surveillance should employ two arms of case ascertainment and case investigation:
- follow-up of infants born to HCV-positive pregnant people and
- follow-up of children <36 months of age who have been tested for hepatitis C.
Collectively, these children should be tested appropriately for hepatitis C, and the epidemiologic link with an HCV-positive birth parent should be established where feasible. If screening for hepatitis C does not occur during every pregnancy, there will inevitably be HCV-positive pregnant people whose infections are not reported to the appropriate HD. To ensure identification of all gestational parent-infant pairs, it is critical to use multiple methods for identifying HCV-positive pregnant people or gestational parents and children <36 months of age who have been tested for hepatitis C.
As resources permit, consider that identification during pregnancy does not currently have public health utility in preventing vertical HCV transmission; however, identification of HCV-positive pregnant people can be leveraged to improve treatment outcomes in the post-partum period to prevent subsequent perinatal HCV exposures. Further, earlier identification of HCV-positive pregnant people or gestational parents can improve HCV testing outcomes in their infants and children, allow for timely communication with pediatric providers regarding the exposure and HCV testing recommendations.
To facilitate identification of HCV-infected infants, perinatal hepatitis C surveillance staff should
- develop perinatal hepatitis C programs that have procedures for active tracking and case management of infants born to HCV-infected gestational parents;
- provide case management guidance to health care practitioners and health care organizations that provide care to infants and children;
- conduct follow-up investigation on any anti-HCV-positive infant with no or unknown HCV detection test, including recommending HCV RNA testing to determine whether the infant has hepatitis C and requires linkage to medical care;
- consider measures to facilitate prenatal HCV screening during each pregnancy in settings where the HCV RNA prevalence is >1% (currently, most settings are unlikely to have an HCV RNA prevalence as low as 0.1%) or HCV RNA prevalence is unknown;
- make positive HCV RNA test results in pregnancy a reportable condition;
- establish links with hospitals and infection control practitioners to facilitate reporting of all births to HCV-positive gestational parents;
- consider requirements to document the maternal HCV infection status on the newborn metabolic screening card, hospital discharge summaries, and birth certificate;
- evaluate if HCV RNA testing in the first year of life of all children born to HCV-infected gestational parents serves as a practical method to improve follow-up;
- establish routine reporting as part of HD case management of all HCV-RNA and anti-HCV test results (positive and negative) from infants; and
- routinely match HCV cases reported to the jurisdiction to birth records. (Some jurisdictions have found low sensitivity with vital records matching [i.e., birth records] and would recommend it as a supplementary rather than sole source for case ascertainment.)
Figure 4-3 illustrates a potential approach for perinatal hepatitis C case ascertainment and classification. Specific case ascertainment and classification procedures vary by jurisdiction based on established processes, but should generally follow the scheme below in accordance with the CDC/CSTE Position Statement for the 2018 perinatal hepatitis C case definition (114, 115).
Figure 4-3. Process for perinatal hepatitis C case ascertainment and classification
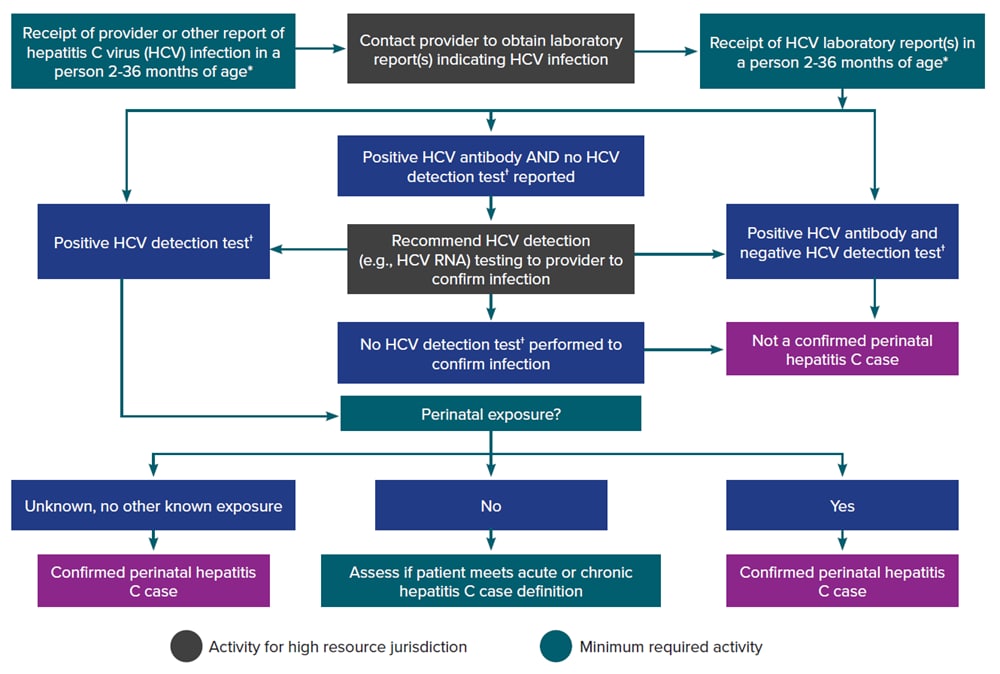
*HCV detection testing includes nucleic acid testing (NAT) for HCV RNA (including qualitative, quantitative, and genotype testing) or testing indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
†Test results among infants <2 months of age should not be used for classification. Cases among children <36 months of age who are known to have been exposed to HCV through health care or otherwise, and not perinatally, should be reported under the 2020 acute and chronic hepatitis C case definitions.
Downloads of this figure: PDF | PPT
Case Investigation
The following elements can inform investigation and management of the HCV-infected pregnant person and infant:
- Demographic information. For the pregnant person, obtain date of birth, current gender, race, ethnicity, residential address (including zip code), and insurance status. For the infant, obtain the date and place of birth, birth weight, sex, race, ethnicity, and insurance status. The contact information of the legal guardian(s) should also be collected.
- Patient and health care provider information. Obtain names of and contact information for the prenatal care provider, infant’s health care provider, and parent(s) or legal guardian(s). Information about adoption or foster-care status should also be collected.
- Delivery information. Document the expected and actual due dates and delivery facilities.
- Diagnostic test results. Obtain documentation of positive HCV test results for both parent and infant. Anti-HCV test results alone do not meet the case definition for perinatal hepatitis C at any age but should still be collected for surveillance and follow-up purposes, including to identify the need for HCV RNA testing of infants exposed at birth.
- Clinical features. For the pregnant person, document the presence of jaundice and whether this is a newly acquired infection or of an existing condition. Most infants with hepatitis C are asymptomatic.
- Epidemiologic link. For the infant, confirm birth to an HCV RNA-positive gestational parent and the absence of other potential exposures beyond perinatal.
- Reporting information. For all cases, obtain the date reported to health jurisdiction, date of diagnosis, date the investigation was initiated, date of first contact with patient and/or health care provider, and date referred for medical evaluation.
- Education and referral for follow-up. Provide education according to national guidelines, to include discussion of perinatal transmission risk, need for appropriate testing for the infant, and current treatment recommendations. No DAAs have been approved for use in pregnancy; AASLD/IDSA guidelines do not recommend treatment during pregnancy (92).
Because stigma against pregnant PWUD could lead to reluctance to seek and continue prenatal care, information collected for public health surveillance should not be used for law enforcement purposes.
Case Investigation Prioritization
Follow-up and investigation of a case of hepatitis C in a pregnant person should occur in pregnancy or as soon as possible thereafter. Successful follow-up might be more likely if contact information is utilized sooner. Further, if treatment as prevention or other interventions are established as standard practice, early identification of HCV-positive pregnant people and timely follow-up will facilitate the prevention of perinatal HCV transmission.
The following situations are considered high-priority for case investigation and follow-up among HCV-positive people of childbearing age with childbearing potential:
- Pregnancy status is unknown
- Co-infected with HIV
- High HCV RNA levels
Case Management
HCV-positive pregnant people should be investigated and followed up in accordance with practices outlined for cases of acute hepatitis C and chronic hepatitis C (see Section 4.6.5). In addition, HCV-positive gestational parents and their infant(s) should be followed through a system that can track case management processes and allow for sharing and/or linking parent and child events. The system should allow each pregnancy to be considered unique and pregnancy-specific data to be captured and maintained. Infants should be monitored in this system at appropriate intervals to ensure appropriate post-birth testing is performed. Viral hepatitis surveillance staff should work with their viral hepatitis prevention coordinator to ensure proper follow-up of infants born to HCV-positive gestational parents.
Perinatal hepatitis C cases should be followed through a minimum of 36 months of age to enable jurisdictions to track spontaneous clearance of infection, progression to chronic infection, clinical evaluation, and treatment/cure. Viral hepatitis prevention coordinators should include information on proper follow-up of these infants in their provider education. HDs should make best efforts to perform some or all the following case-management activities depending on available resources:
- Ensure testing of exposed infants.
- Children born to an HCV-positive gestational parent should be tested according to AASLD/IDSA HCV clinical guidelines. Pediatricians should be informed regarding the HCV exposure and testing guidelines, as appropriate.
- Children with a positive anti-HCV result prior to 18 months of age should receive HCV RNA testing (if not automatically reflexed).
- Provide parents and caregivers with education regarding the potential for vertical transmission.
- Counsel parent(s) or legal guardian(s) about HCV vertical transmission and testing recommendations for their infant.
- Provide information on HCV transmission risk to the infant, testing recommendations, and directed medical care.
- Refer HCV-positive people for medical evaluation.
- Post-partum HCV curative treatment for HCV-positive gestational parents should be promptly provided by a medical provider according to treatment guidelines, helping reduce disease progression and the risk of future perinatal transmission.
- HCV-positive gestational parents should be linked to other services as needed.
- Children with a positive HCV RNA result performed at >2 months of age or positive anti-HCV result performed >18 months of age should be evaluated (by referral or consultation, if appropriate) to
- verify the presence of HCV infection and
- assess for severity of disease and possible treatment according to current practice guidelines in consultation with, or by referral to, a specialist knowledgeable in this area.
Siblings born to the same gestational parent should be tested if they have not been previously tested, and if positive, reported to CDC according to the appropriate case definition (i.e., children ≥36 months of age should be evaluated based on case definitions for acute and chronic infection).
Case Reporting and National Notification
HCV infection during pregnancy should be a reportable event to the HD. Although no specific NNDSS event exists for HCV infection during pregnancy, CDC recommends classifying these cases as acute or chronic using the appropriate event code (Table 1-2) in accordance with the CDC/CSTE case definitions, and transmitting the pregnancy status to NNDSS for all cases among people of childbearing age with childbearing potential to allow for national tracking. At the jurisdiction-level, there may be a separate classification category specifically for hepatitis C during pregnancy. Cases of perinatal hepatitis C are nationally notifiable to CDC and are submitted using a condition-specific event code (Table 1-2). A case classified as perinatal hepatitis C can be additionally enumerated as a confirmed case of chronic hepatitis C if a positive HCV viral detection test (e.g., HCV RNA) is obtained after the case is >36 months of age. See Section 4.5 for more information on hepatitis C case reporting and national notification.