Hepatitis B Surveillance Guidance
The information on this page reflects the 2024 acute and chronic HBV case definition updates. FOR 2023 HBV CASE CLASSIFICATION, use: 2023 Case Classification_Full Guidance PDF
- Background
- Cases and Clusters of Potential Public Health Importance
- Interpretation of Laboratory Test Results
- Recommended Reportable Laboratory Markers
- Case Reporting and National Notification
- Surveillance of Acute and Chronic Hepatitis B
- Surveillance of Hepatitis B During Pregnancy and Perinatal Hepatitis B
Background
Hepatitis B is a disease caused by the hepatitis B virus (HBV) that can be self-limited for some and lifelong for others. HBV is transmitted through the blood or bodily fluids of an infected person. In the United States, injection drug use (IDU) and having multiple sexual partners are the first and second most common risk behaviors or exposures reported for acute hepatitis B, respectively (3). Approximately 50–70% of people with acute hepatitis B are not symptomatic (45), resulting in many undiagnosed and unreported infections. HBV is highly transmissible and infectious on environmental surfaces for at least 7 days (46).
The epidemiology of hepatitis B in the United States has evolved since the hepatitis B vaccine first became available in 1982 (47). Declines in acute hepatitis B incidence following the expansion of vaccination recommendations ceased during 2010–2019. Decreases occurred in 2020 and 2021 during which disruptions caused by the COVID-19 pandemic made it difficult to determine the impact of prevention efforts (3). The incidence of acute hepatitis B is highest among non-Hispanic White people and non-Hispanic Black people (3). National chronic hepatitis B prevalence and death rates have remained relatively stable (1, 3).
During January 2017–March 2020, approximately 0.2% of the non-institutionalized US population, representing approximately 660,000 people, were estimated to be living with chronic hepatitis B (1). The prevalence of chronic hepatitis B was highest among non-US-born people and those of Asian/Pacific Islander descent (1). Approximately 50% of people living with chronic hepatitis B were unaware of their infection status (1). Such people could unknowingly transmit their infection to others and are at risk for developing chronic liver disease.
There are clinical guidelines by the American Association for the Study of Liver Diseases (AASLD) for the prevention, diagnosis, and treatment of chronic hepatitis B (48). Several antiviral medications are available to effectively lower HBV DNA levels and slow the progression of liver disease; however, hepatitis B is not yet curable.
Recommendations for universal adult hepatitis B screening were released by the CDC in (49). They recommend that all adults >18 years of age be screened at least once. All pregnant people are recommended for universal hepatitis B screening, preferably during the first trimester and regardless of vaccination status or testing history, because of the risk for perinatal transmission. People being tested for the first time should be tested using the “triple panel” – which includes hepatitis B surface antigen (HBsAg), total antibody to hepatitis B core antigen (anti-HBc), and hepatitis B surface antibody (anti-HBs) – to identify people who have current HBV infection, have resolved acute HBV infection and may be at risk for reactivation, are susceptible and need vaccination, or are vaccinated with immune protection. Infants who are perinatally exposed to HBV should receive the first dose of hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth, complete the vaccine series, and receive post-vaccination serologic testing (PVST) for HBsAg and anti-HBs during 9–12 months of age and 1–2 months after the final dose of the vaccine. Without preventive interventions, chronic infection develops in approximately 90% of infected infants compared with 25%–30% of children who acquire HBV infection during 1–5 years of age and about 10% of people infected at >5 years of age (50, 51).
All unvaccinated people with ongoing risk behaviors or exposures should be tested for hepatitis B periodically while risk behaviors or exposures persist. People for whom HBV exposure is suspected should receive timely post-exposure prophylaxis and immediate testing, which can prevent HBV infection and interrupt transmission. In 2022, the ACIP recommended hepatitis B vaccination for all adults 19–59 years removing the need for risk factor screening among this age group (52). Infants and people <19 years of age regardless of risk factors for hepatitis B and adults >60 years of age with risk factors for hepatitis B are also recommended to receive vaccination. Adults aged ≥60 years without known risk factors for hepatitis B may receive vaccination.
The purpose of this section is to provide guidance to jurisdictions as they implement and improve hepatitis B surveillance. It contains information regarding reporting requirements, collection of relevant laboratory data, and case investigation. Given that current systems for the surveillance and follow-up of hepatitis B cases differ by jurisdiction, the standards outlined in this document are designed to provide models for best practices, recognizing that not every jurisdiction can meet those standards with available resources.
Cases and Clusters of Potential Public Health Importance
Jurisdictions should review and analyze hepatitis B data regularly to identify cases and clusters of hepatitis B that merit further investigation. When resources are limited, these should be prioritized for investigation based on the degree of public health importance. The following are examples of high priority cases and clusters:
- People of childbearing age who are or have the potential to become pregnant, indicating the potential for perinatal transmission
- Children ≤24 months of age to detect perinatal transmission
- People in age and demographic groups for whom infection may be acute due to recent transmission, including those
- ≥70 years of age (indicating possible health care-associated transmission)
- People who were previously vaccinated to characterize possible vaccine failures (see Section 1.10 Case Investigation)
- People born after 1990 to distinguish between failure of vaccine and failure to vaccinate
- People receiving hemodialysis with evidence of acute hepatitis B (including those with test conversions)
- People lacking typical behavioral risk behaviors or exposures for hepatitis B (e.g., IDU) who have evidence of acute infection (including test conversions) to identify other potential causes of HBV transmission (e.g., health care-associated exposures) (information on investigation of health care-associated outbreaks can be found on the CDC DVH Viral Hepatitis Outbreaks website)
- People with other indicator(s) of possible acute or recent infection, including those
- with elevated ALT or jaundice;
- with positive immunoglobulin class M antibody to hepatitis B core antigen (anti-HBc IgM);
- with recent or current IDU history;
- who were tested at locations frequented by people at high-risk for acute infection (e.g., STI and HIV clinics, SSPs, correctional facilities, and medication-assisted treatment for opioid use disorder [MAT] centers); or
- who were in a residential facility or custodial care, including long-term care or correctional facilities, for ≥6 months prior to the onset of symptoms.
Interpretation of Laboratory Test Results
A description of hepatitis B laboratory markers can be found in Appendix B.
Understanding Changes in Biomarkers During Disease Progression
Understanding the changes in HBV biomarkers over the course of a person’s infection and recovery is key to interpreting the test results. Figure 3-1 and Figure 3-2 depict the typical biomarker changes over the course of hepatitis B disease.
Figure 3-1. Typical serologic course of acute hepatitis B to recovery

Weeks after Exposure
Downloads of this figure: PDF | PPT
Figure 3-2. Typical serologic course of the progression to chronic hepatitis B

Weeks after Exposure
Downloads of this figure: PDF | PPT
Acute, resolved, and chronic hepatitis B
Approximately 90% of people >5 years of age with acute hepatitis B will spontaneously clear their infection (50, 51). People with resolved hepatitis B will remain positive for total anti-HBc and develop anti-HBs that protect against future HBV infection (Figure 3-1). Chronic hepatitis B is defined as an HBV infection lasting >6 months. During the typical course of chronic infection, the total anti-HBc and HBsAg markers will always be present, whereas anti-HBc IgM will disappear (Figure 3-2). Hepatitis B e antigen (HBeAg) and hepatitis B e antibody (anti-HBe) are variably present. HBV DNA levels vary during the course of chronic infection. Any detectable HBV DNA level is considered positive for surveillance purposes.
Isolated total anti-HBc positive
A person with a positive total anti-HBc with corresponding negative HBsAg and anti-HBs results is considered to be isolated total anti-HBc positive. A small fraction of these people could have low level chronic viremia, also known as occult hepatitis B, in which HBsAg is absent in the serum but HBV DNA is detectable (53) (Table 3-1). Cases of occult hepatitis B may be missed through surveillance in the absence of a provider report indicating occult infection or in the absence of total anti-HBc and HBV DNA results. To determine if occult hepatitis B is present, those who are isolated total anti-HBc positive should be tested for the presence of HBV DNA.
People with mutations in HBsAg that cannot be detected by current serologic assays may present with a negative HBsAg result despite high blood levels of HBV DNA. Some laboratories have the capacity to detect HBsAg mutants. Any HD interested in determining which laboratories can detect HBsAg mutants should follow-up with the major laboratories that perform HBsAg testing in their jurisdiction.
Hepatitis B reactivation
People with a history of hepatitis B – either inactive chronic hepatitis B or resolved acute hepatitis B – can experience hepatitis B reactivation. Hepatitis B reactivation is the loss of hepatitis B immune control, which can be followed by a hepatitis flare characterized by ALT elevation (increase to greater than 3 times the baseline level and >100 IU/L with or without symptoms; in some cases, illness can be severe and result in death (48). In general, people with inactive chronic hepatitis B (i.e., those positive for HBsAg) are at greater risk for reactivation than are those with resolved hepatitis B (i.e., those negative for HBsAg and positive for total anti-HBc and anti-HBs). People at greatest risk of hepatitis B reactivation include those
- undergoing cancer chemotherapy,
- receiving immunosuppressive therapy (particularly anti-B cell therapy),
- with HIV infection who have discontinued antiretroviral drugs with activity against HBV (e.g., tenofovir),
- undergoing solid organ or bone marrow transplantation, and
- co-infected with hepatitis C virus (HCV) who are undergoing treatment with direct-acting antivirals (DAAs).
Among people with previously inactive chronic hepatitis B (i.e., those positive for HBsAg and total anti-HBc), laboratory evidence of reactivation includes meeting any one of the following criteria:
- a ≥100-fold increase in HBV DNA compared to the baseline level,
- HBV DNA ≥1,000 IU/mL in a patient with previously undetectable level, or
- HBV DNA ≥10,000 IU/mL if the baseline level is not available (48).
Among people with resolved acute hepatitis B (i.e., negative for HBsAg and positive for total anti-HBc and anti-HBs), laboratory evidence of reactivation includes meeting either of the following criteria:
- HBV DNA is now detectable or
- HBsAg test conversion occurs (negative HBsAg to positive HBsAg) (48).
People with hepatitis B reactivation are frequently positive for anti-HBc IgM. People with previously resolved infection who reactivate can have clinical signs and symptoms while also being transiently positive for anti-HBc IgM, therefore, mimicking acute infection. If it is determined that the case under investigation has test results known to be due to reactivation (e.g., a prior history of acute or chronic hepatitis B), the positive anti-HBc IgM test result should not be used for case classification purposes.
Obtaining a clinical history from the patient’s provider and/or checking the surveillance system or registry might provide clarification. A history of acute or chronic hepatitis B can help distinguish between a hepatitis B reactivation case (history of hepatitis B) and a newly diagnosed acute or chronic hepatitis B case (no history of hepatitis B). History of acute or chronic hepatitis B includes those that were previously reported to and documented in a jurisdiction’s surveillance system, were reported by a separate jurisdiction as having such a history, or a provider report of prior infection.
If reactivation is identified in a previously reported probable or confirmed acute hepatitis B case in a prior reporting year, and the person meets chronic case classification criteria with the latest report, this case should be submitted as a new chronic hepatitis B case. If reactivation is identified in a previously reported probable or confirmed chronic hepatitis B case, this case should not be counted again; however, reactivation may be noted in the jurisdiction’s hepatitis B surveillance database if the jurisdiction chooses to do so.
Interpreting Hepatitis B Laboratory Results
Many jurisdictions have regulations requiring laboratories to report all positive HBsAg, HBeAg, HBV DNA, and anti-HBc IgM laboratory results to the HD while a subset might also routinely receive positive total anti-HBc and anti-HBs results.
Additionally, some HDs might receive negative hepatitis B laboratory results, which are useful for determining false-positive results and monitoring patients through their infection and recovery. In jurisdictions that receive laboratory results performed as part of a reflex test panel, implementation of the initial screening triple panel (HBsAg, total anti-HBc, and anti-HBs) may result in more complete reporting of hepatitis B laboratory results among people with HBV infection. Table 3-1 shows how to interpret the combinations of laboratory results frequently available in hepatitis B test panels, following the biomarker changes over the course of disease as shown in Figure 3-1.
Table 3-1. Interpretation of hepatitis B laboratory results
| HBsAg | Total anti-HBc | Anti-HBc IgM | Anti-HBs | HBV DNA | Possible Interpretation* |
|---|---|---|---|---|---|
| – | – | – | – | – | Never infected; susceptible if never vaccinated or vaccine failure |
| + | – | – | – | + or – | Early acute infection (if HBV DNA is positive); transiently positive for HBsAg after vaccination (if HBV DNA is negative)† |
| + | + | + | – | + | Acute infection |
| – | + | + | + or – | + or – | Acute resolving infection; “window period” if anti-HBs is negative |
| – | + | – | + | – | Recovered from past infection and immune |
| + | + | – | – | + | Chronic HBV infection |
| – | – | – | + | – | Immune from vaccination; passive anti-HBs transfer after hepatitis B immune globulin administration |
| – | + | – | – | + or – | Isolated total anti-HBc positive‡ |
| – | + or – | – | + or – | + | Occult HBV infection§ |
| + or – § | + | + or – | + or – | + | Possible HBsAg mutant infection |
Table modified from https://www.cdc.gov/mmwr/volumes/67/rr/pdfs/rr6701-H.PDF.
Abbreviations: – = negative; + = positive; anti-HBc = antibody to hepatitis B core antigen; anti-HBs = antibody to hepatitis B surface antigen; HBsAg = hepatitis B surface antigen; HBV DNA = hepatitis B virus deoxyribonucleic acid; IgM = immunoglobulin class M.
*Ingestion of high levels of biotin can significantly interfere with certain commonly used biotinylated immunoassays and cause false-positive or false-negative laboratory test results. The US Food and Drug Administration (FDA) is investigating thresholds associated with false-positive and false-negative tests. This section will be updated as more information becomes available. Reference: https://www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication.
†People who receive hepatitis B vaccine might be transiently positive for HBsAg, with reports of transient positivity 18 days post-vaccination (56). Retesting of patients who are positive for HBsAg shortly after hepatitis B vaccination at a later time is needed to determine the true HBV infection status.
‡Could result from:
- Loss of anti-HBs after past resolved infection. HBV DNA is negative.
- False-positive total anti-HBc, i.e., susceptible. HBV DNA is negative. To resolve the ambiguity of a false-positive total anti-HBc result, test a follow-up sample 4–8 weeks later. If found positive, interpret as a resolved infection. If negative, interpret as false-positive.
- Passive maternal transfer of total anti-HBc to infant born to a HBsAg-positive gestational parent for up to 24 months. HBV DNA is negative.
- Occult HBV infection. HBV DNA is positive, typically at low levels. Anti-HBs might or might not be positive.
- HBsAg mutant infection. HBV DNA is positive, typically at high levels. Anti-HBs might or might not be positive.
§HBsAg mutants will not be detectable if testing was performed using an older assay that cannot detect HBsAg mutants. HBsAg mutant strains can be detected by some HBsAg assays that first became available in the United States in 2015, including Abbot ARCHITECT instrument, ETI-MAK-2 PLUS, and Siemens Advia Centaur XP or XPT instrument. Though specimens should be tested using an assay that can detect HBsAg mutants, older HBsAg assays that cannot detect HBsAg mutants remain available. Reference: Apata I W, Nguyen D B, Khudyakov Y, et al. Hepatitis B virus mutant infections in hemodialysis patients: A case series. Kidney Medicine 2019; 1(6): 347-353. DOI: https://doi.org/10.1016/j.xkme.2019.07.011.
Downloads of this figure: PDF | PPT
Recommended Reportable Laboratory Markers
The following laboratory markers are recommended for reporting to public health, as they can aid in case ascertainment, case classification, and monitoring care continua for hepatitis B:
- Positive HBsAg
- Positive/detectable HBV DNA (including quantitative, qualitative, and genotype testing)
- Positive HBeAg
- Positive anti-HBc IgM
- If any of the above positive results are reported, also report the following:
- Pregnancy status
- Concurrent ALT and total bilirubin result
- Other hepatitis serological results (e.g., hepatitis A, hepatitis C, hepatitis D, and/or hepatitis E)
- Total anti-HBc* results
- Negative HBsAg
- Negative/undetectable HBV DNA† results
* Total anti-HBc is detectable, on average, approximately 5 weeks post-HBV exposure, remains detectable indefinitely following exposure, and indicates past or current infection. In the presence of total anti-HBc, a positive HBsAg, HBeAg, or anti-HBc IgM result is a more reliable indication of current or recent infection while negative total anti-HBc results can be used to determine that a person is not a case. Jurisdictions that receive total anti-HBc laboratory results can use these results to clarify a person’s HBV infection status and confirm chronic cases in conjunction with evidence of positive tests for HBsAg or HBeAg.
† Jurisdictions are encouraged to require reporting of negative/undetectable HBV DNA results as these results can be a proxy indicator that people living with hepatitis B have been linked to care. This information can support accurate longitudinal surveillance and measurement of hepatitis B care continua.
Case Reporting and National Notification
Cases of acute, chronic, and perinatal hepatitis B, and hepatitis B during pregnancy should be reported to HDs as specified by state, territorial, or local regulations. Acute, chronic, and perinatal hepatitis B are nationally notifiable conditions (5). Hepatitis B cases are identified using an event code corresponding to the hepatitis B condition (Table 1-2). Data are sent weekly or more frequently, depending on the infrastructure of the jurisdiction sending the data. Cases might be re-classified or removed as needed after the initial transmission to CDC, as long as the changes occur before surveillance data are finalized each year.
Surveillance of Acute and Chronic Hepatitis B
Uses of Surveillance Data
Surveillance Case Definitions
Case Ascertainment
Case Investigation
Case Reporting and National Notification
Surveillance Activities for Chronic Hepatitis B
Considerations for Hepatitis B Cases who were Transplant Recipients
Monitoring Infection Trends and Disease Outcomes Using a Person-Level Database and Supplemental Data Sources
Background
The national incidence of acute hepatitis B dramatically declined after incremental recommendations for vaccinating people at-risk for infection and severe outcomes were released beginning in 1982 (47) and for infants and children in 1991 (55). The number of acute hepatitis B cases reported each year in the United States has remained relatively stable during 2014–2019 but decreased abruptly in 2020 and 2021 (3). The decrease in reported cases could be due to prevention efforts as well as disruptions of the COVID-19 pandemic. IDU and having multiple sexual partners are major risk behaviors associated with acute hepatitis B in the United States, and incidence is highest among non-Hispanic White people, non-Hispanic Black people, and those 30–59 years of age (3).
In the United States, chronic hepatitis B occurs primarily among people born in countries with intermediate or high hepatitis B prevalence, where the primary mode of transmission is perinatal transmission. Chronic hepatitis B occurs in about 1.0% of non-US-born adults (1). During January 2017–March 2020, approximately 660,000 people were estimated to be living with chronic hepatitis B in the United States (1).
CDC has provided guidelines for hepatitis B screening among all adults >18 years of age at least once during a lifetime and pregnant people during each pregnancy (49). Periodic testing among susceptible people with ongoing risks for exposures is recommended while risks for exposures persist. Undiagnosed hepatitis B cannot be detected using traditional surveillance methods. Improving hepatitis B surveillance by improving screening is an important component of national and jurisdictional strategies for the prevention and control of hepatitis B.
Uses of Surveillance Data
Acute and chronic hepatitis B surveillance data can be used to inform and improve public health interventions in the following ways:
- Monitoring trends in disease incidence and determining risk behaviors or exposures. Acute hepatitis B surveillance data should be analyzed at regular intervals by person, place, and time to monitor disease incidence. Risk factor information should be analyzed to monitor disease transmission patterns and identify groups at higher risk for infection.
- Identifying outbreaks. An outbreak is defined as the occurrence of more cases of disease than expected in a given area or among a specific group of people over a particular time period. Detailed guidance on viral hepatitis outbreaks, including examples of hepatitis B outbreaks, can be found on the CDC DVH Viral Hepatitis Outbreaks website.
- Assessing missed opportunities for prevention.
- Patients whose infection source was reported as being a household or sexual contact should be investigated to determine whether they should have been vaccinated when the source case was identified. Potential barriers to administering post-exposure prophylaxis should be explored to mitigate future missed opportunities for prevention.
- Surveillance data can be used to provide information on cases occurring among adults at high risk for infection, creating opportunities to provide education and awareness to the health care community and the public about the importance of vaccinating high-risk populations as recommended by ACIP.
- Missed opportunities for vaccination should also be assessed for cases occurring among people born after 1990. Understanding the frequency and characteristics of these cases enables monitoring of the effectiveness of routine childhood vaccination programs and identification of barriers to childhood vaccination.
- Assessing the frequency and causes of vaccine failure. When available, vaccination history should be obtained. Though vaccine failure is rare, any case in a person who was previously vaccinated requires additional investigation to identify potential instances of vaccine failure. Where available, jurisdiction immunization registries can provide valuable information for such investigations Section 1.10. Health care professionals or public health authorities who have questions on these cases should contact CDC’s DVH at viralhepatitis@cdc.gov. Reporting and investigating these cases through surveillance is important for informing vaccination policies and education.
- Tracking cases of chronic hepatitis B. Surveillance systems and databases that track chronic hepatitis B cases can aid in monitoring trends in the prevalence of chronic infection.
- Understanding the burden of hepatitis B in the community. Person-based longitudinal databases can provide a better understanding of the burden of hepatitis B in the community. Such databases can help
- determine whether infections have resolved or reactivated;
- identify probable cases that need additional testing for diagnosis;
- identify health-related disparities;
- facilitate identification of HBV-infected pregnant people for enrollment in the Perinatal Hepatitis B Prevention Program (PHBPP);
- facilitate monitoring of perinatal hepatitis B;
- monitor the movement of cases in or out of the jurisdiction; and
- track the occurrence of related adverse health outcomes.
- Public health management of people with chronic hepatitis B and their contacts. Surveillance data can be used to identify and follow-up on chronic hepatitis B cases (especially among those who were recently diagnosed), link them to appropriate medical care and harm reduction services, and ensure contacts are protected and/or referred to care or testing, as appropriate.
Surveillance Case Definitions
Table 3-2 depicts the surveillance case definition for acute hepatitis B, adopted by CSTE and CDC in 2024 (56, 57). See Appendix C for classification scenarios of cases of acute hepatitis B.
Table 3-2. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for acute hepatitis B, 2024
| Criteria Type | Criteria |
|---|---|
| Age |
|
| Clinical |
|
| Confirmatory Laboratory* | Tier 1
Tier 2
|
| Presumptive Laboratory* |
|
| Case Status | Classification |
| Confirmed Acute* |
|
| Probable Acute* |
|
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate.
†If information on HBsAg test method is available and HBsAg confirmatory neutralization was performed as recommended, HBsAg positive by confirmatory neutralization.
Download of this table: PDF
Table 3-3 specifies the surveillance case definition for chronic hepatitis B, adopted by CSTE and CDC in 2024 (57, 58). See Appendix C for classification scenarios of cases of chronic hepatitis B.
Table 3-3. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for chronic hepatitis B, 2024
| Criteria Type | Criteria |
|---|---|
| Age |
|
| Clinical |
|
| Confirmatory Laboratory * |
|
| Presumptive Laboratory* |
|
| Case Status | Classification |
| Confirmed Chronic*‡ |
|
| Probable Chronic*‡ |
|
Download of this table: PDF
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate.
†If information on HBsAg test method is available and HBsAg confirmatory neutralization was performed as recommended, HBsAg positive by confirmatory neutralization.
‡A confirmed or probable acute hepatitis B case may be additionally enumerated as a new confirmed chronic hepatitis B case if a positive HBV viral detection test is reported 6 months or longer after acute case onset or, if asymptomatic, after the initial positive test result.
All Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories should be performing HBsAg confirmatory neutralization testing in accordance with CLIA regulations and the signal-to-cutoff values criteria listed in the instructions for use insert of the laboratory test assay. See Appendix D for more information on the HBsAg testing sequence. Therefore, HDs should not need to investigate every positive HBsAg result to determine whether confirmatory neutralization was performed. However, if a HD receives a negative or indeterminate HBsAg confirmatory neutralization result, the initial screened positive HBsAg result should not be used to count a person as a case.
False-positive HBsAg and HBeAg test results are possible. This can be due to multiple factors, including hepatitis B vaccination in the prior 30 days (e.g., can be longer if the person is a hemodialysis patient) and certain health conditions. If a false-positive result is suspected, jurisdictions should consider other available test results, such as the total anti-HBc result, to aid with interpretation. If results are determined to be false-positive, they should not be used to classify cases as confirmed or probable.
Multiple hepatitis B laboratory tests may be performed simultaneously on the same patient specimen as part of a “hepatitis panel.” Testing performed in this manner may lead to seemingly discordant results, e.g., negative HBsAg and positive HBV DNA. For the purposes of these case definitions, any positive result among the three viral detection tests (HBsAg, HBeAg, and HBV DNA) is acceptable for case classification, regardless of other testing results. Negative HBeAg results and HBV DNA levels below the positive cutoff level do not rule out HBV infection.
HBV DNA results might be reported as below the lower limit of quantification (e.g., < 15 IU/mL, etc.) yet simultaneously indicate that HBV DNA was detected. HBV DNA results that are above the limit of detection but below the lower limit of quantification should be considered “detectable” or “positive” for surveillance classification purposes.
The serologic course of acute hepatitis B lasts for <6 months. Therefore, the clinical and laboratory evidence used to classify a case as acute hepatitis B must have occurred within a time that is no longer than 6 months to meet classification requirements.
The “absence of a more likely diagnosis” clause of the acute clinical criteria can be applied when there is documentation of an alternative diagnosis that would more likely explain the presence of clinical evidence (i.e., a provider report of jaundice, total bilirubin >3.0 mg/dL, or ALT >200 IU/L). If there is documentation from the patient’s health care provider explaining that the clinical evidence is due to another reason other than acute hepatitis B, the patient should not be evaluated under the acute hepatitis B case definition.
A case meeting the requirements for being classified as an acute hepatitis B case might also meet requirements for being classified as a chronic hepatitis B case. In this circumstance, unless there is evidence that the case is not acutely infected (e.g., hepatitis B reactivation), the case should only be classified as an acute case. If after 6 months from the initial event date, the case continues to have test results reported that indicate current HBV infection, they can then be additionally classified as a chronic hepatitis B case. People with hepatitis B reactivation should not be counted as new acute hepatitis B cases. People with hepatitis B reactivation may be counted as new confirmed chronic hepatitis B cases if they were not previously classified and reported as such.
Since hepatitis D only occurs in the presence of HBV infection, reports of hepatitis D without known HBV infection should be investigated as suspected cases of hepatitis B.
Case Ascertainment
The primary method to ascertain suspected cases is through investigation of reports from clinical laboratories, health care facilities, and health care providers suggestive of hepatitis B. Rules or regulations requiring that facilities and providers report hepatitis B to public health agencies vary by jurisdiction. See Section 1.6 and Section 3.4 for information on the recommended reporting requirements for hepatitis B.
Additional sources of information include medical records, hospital discharge databases, death certificates, and birth certificates. Section 5.4 provides more information on these data sources. Figure 3-3 illustrates a potential approach for acute and chronic hepatitis B case ascertainment and classification.
Specific procedures can vary by jurisdiction, but should generally follow the scheme below, in accordance with the CDC/CSTE Position Statement for the 2024 acute and chronic case definitions (56-58). Children <24 months of age and born in the United States to a gestational parent with documented evidence of HBV infection should be classified and reported using the 2017 perinatal hepatitis B case definition (59, 60), unless there is evidence that exposure occurred via a non-perinatal mechanism (e.g., health care-acquired). See Section 3.7.4 for case ascertainment guidance of perinatal hepatitis B cases.
Laboratory Reporting
Laboratory reporting of HBV infection is required in all states for which acute and/or chronic hepatitis B are reportable. While case-defining infection markers (e.g., positive HBsAg or anti-HBc IgM) are reportable in most jurisdictions, regulations vary regarding which markers should be reported (e.g., any or all positive indicators within the panel, only positive results of selected biomarkers, or selected combinations of markers).
Some jurisdictions require reporting of negative hepatitis B laboratory results for some or all the infection markers or when accompanied with positive hepatitis B laboratory results. Receiving negative hepatitis B laboratory results or complete reporting of all tests in the hepatitis panel allows public health officials to interpret results more accurately. However, this also requires more sophistication in information systems to efficiently send, process, and utilize the information received.
Health Care Facility and Provider Reporting
Many states require health care facilities and providers to report hepatitis B diagnoses.
Figure 3-3. Process for classifying cases of hepatitis B as acute and chronic
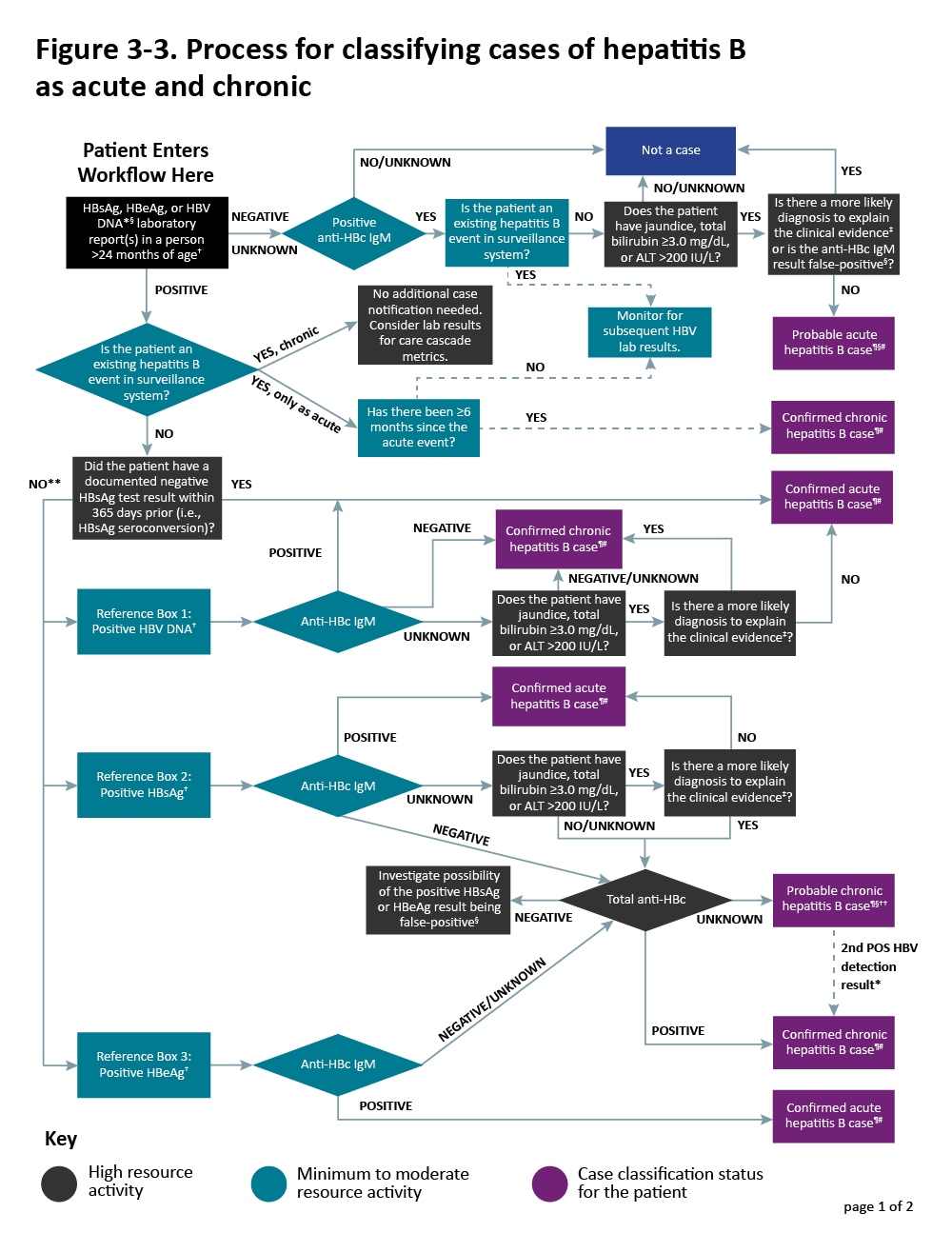
Case Investigation
The original report may be sufficient to classify a case as an acute or chronic infection. Resource limitations may not allow all chronic cases to be investigated in the same way as acute cases. Additional investigation may be necessary depending on the priority level of the case. The level of investigation will depend on the situation, the objectives, and the available resources. The following is a description of the type of information that should be collected during case investigations:
Information from the Laboratory
Newly reported positive anti-HBc IgM, HBsAg, HBeAg, and HBV DNA laboratory results should be reported to the HD. Concurrent ALT and total bilirubin results reported with positive hepatitis B laboratory results can be helpful in identifying cases that might be acute. Total anti-HBc indicates past or current infection, and in its presence, a positive HBsAg, HBeAg, or anti-HBc IgM result is more likely to be a true positive.
Information from the Provider or Medical Records
Medical records can provide the following types of information:
- Demographic information. Includes name, date of birth, sex at birth, current gender, race, ethnicity, country of birth, and residential address (including zip code).
- Clinical features. Includes reason for testing, illness onset date, clinical signs (jaundice) and symptoms (if available*), hospitalization status and date of death (if applicable), and whether an alternate diagnosis is suspected. HDs should inquire about the potential of past infection to confirm whether current clinical features are due to a newly acquired infection. The medical record may also provide evidence of chronic liver disease.
*Includes fever, headache, malaise, anorexia, nausea, vomiting, diarrhea, and abdominal pain. - Pregnancy status. Pregnancy status should be checked for all people of childbearing age with childbearing potential. HBV-infected pregnant people should be referred to the PHBPP to ensure their infants receive appropriate post-exposure management according to ACIP recommendations. Children born to an HBV-infected gestational parent should be tested for infection, and if infected, classified according to the CDC/CSTE perinatal hepatitis B case definition.
- Diagnostic test results. If additional laboratory testing (e.g., ALT levels, total bilirubin levels, and results from a hepatitis panel) are needed to classify the case, HD staff may work with the provider to order/obtain these test results.
- Risk behaviors or exposures. Includes history of IDU, sexual and household exposures, experience of homelessness, recent medical procedures, hemodialysis, incarceration, and residence in a long-term care facility.
- Vaccination information. Vaccination history may be obtained from the patient’s medical provider or from the jurisdiction’s immunization registry. Note that recent hepatitis B vaccination can cause transient HBsAg positivity for up to 18 days post-vaccination (54). Retesting is needed to determine the true HBV infection status in patients who tested positive for HBsAg shortly after hepatitis B vaccination.
Information from the Patient
All patients with acute hepatitis B should be contacted for an interview using the jurisdiction-specific case investigation form. If resources are limited, at a minimum, all patients who are classified as “confirmed” per the CDC/CSTE case definition and those flagged as having public health importance (Section 3.2) should be interviewed. Decisions to contact the patient are often jurisdiction-specific and depend on available resources. In many situations, patient contact is reserved for those cases deemed highest priority for preventing further transmission or for referral for additional care and treatment, as needed.
The patient interview should ideally include the following components:
- Risk behaviors or exposures. This allows identification of a potential source or presence of risk behaviors or exposures for infection during the 60–150 days prior to symptom onset. For chronic cases, if it is determined that the person has current risk behaviors or exposures for ongoing transmission or was identified as part of a cluster of cases, additional information relevant to risk might be prioritized.
- Education and referral for follow-up. Newly diagnosed acute and chronic hepatitis B patients should be advised on how to prevent transmission to others. Patients should also be referred for hepatitis B-directed medical care and recommended to receive vaccination against hepatitis A, if indicated.
- Identification of contacts requiring post-exposure prophylaxis and testing. If resources allow, contacts should be identified and testing, post-exposure prophylaxis, counseling, and linkage to care coordinated, as appropriate. Information regarding hepatitis B vaccination and prophylaxis can be found on the Hepatitis B ACIP Vaccine Recommendations website.
Special Considerations When Investigating Certain Populations or Settings at Risk for Rapid Disease Transmission
Considerations when investigating hepatitis B cases among certain populations at risk for rapid transmission are provided in Section 1.10.
Case Investigation Prioritization
Providers are required to report acute infections directly to the HD, and laboratories should provide HDs with hepatitis B test results electronically. The automated collection of hepatitis B laboratory results will, in many jurisdictions, lead to a high volume of reporting. Many HDs might lack the resources needed to conduct investigations for all acute cases. If resources allow, automate the collection of ALT and total bilirubin results through electronic laboratory reporting (ELR) or electronic medical record (EMR) reporting, and prioritize data collection to confirm those cases with abnormal results. Jurisdictions can also consider the following when prioritizing case follow-up:
- Semi-automated/preliminary collection of risk data combined with more targeted follow-up on cases without identified risk history
- Demographic groups that might be at higher risk for acquiring or transmitting infection
- Pregnant people
- Elderly patients (e.g., >70 years of age)
- Cases that might represent emerging risks
- People infected with HIV, hepatitis C virus (HCV), or STIs
- Groups where infection is unexpected
- Children and adult cases who were born after 1990
- Cases who are documented to have received hepatitis B vaccination
- Target populations based on specific settings within a particular jurisdiction
- SSPs or SUD treatment facilities
- Correctional facilities
- Retirement/nursing facilities
- Providers of people experiencing homelessness
- Areas where known risk behaviors are occurring, or rates of newly reported infections are increasing
- Ease of data collection
- People tested at public health clinics
- Supplement case surveillance data with data sources identifying populations at high risk and the evolving epidemiology of acute infections
- SAMHSA/state drug use, overdose, and EMS data
- HIV, HCV, and STI incidence data to identify coinfections
- Ongoing outbreak and cluster investigations, if applicable
- Hospital discharge data
- Syndromic surveillance data on IDU-related emergency department care
Considerations for Conducting a Chronic Hepatitis B Case Investigation
Conducting an investigation on a chronic hepatitis B case can involve the following considerations:
- Check the jurisdiction’s hepatitis B registry/surveillance system to ensure the case is newly reported and not previously documented.
- Review the information in the initial report to determine if the case potentially falls within a group prioritized for investigation, such as those outlined in Section 3.2. At a minimum, pregnancy status should be checked for all people with chronic hepatitis B who are of childbearing age with childbearing potential; reports of HBV-infected pregnant people should be shared with the PHBPP.
- When possible, contact the health care provider and/or review medical records to obtain additional information to help prioritize which cases should receive a patient interview.
- If the case has one of the risks for hepatitis B reactivation outlined in Section 3.3 under the subsection “Understanding Changes in Biomarkers during Disease Progression” (e.g., the patient is hepatitis C co-infected and is receiving DAA treatment), consider follow-up with the health care provider to ensure the patient receives medical management according to clinical guidelines (61).
- For patients who are interviewed, collect relevant demographic and risk history information using the jurisdiction-specific case report form.
- Investigate likely health care exposures according to the jurisdiction’s procedures, ideally in collaboration with the health care-associated infection team.
- Provide patient education about ways to avoid the spread of infection to others and ways to avoid further harm to the liver.
- Educate long-term sexual contacts and people who have had direct exposure to the patient’s blood about HBV transmission and the need to be tested for hepatitis B if they are not known to be immune or infected. If a contact is susceptible, they should complete the hepatitis B vaccine series; contacts deemed unlikely to return for test results should be vaccinated when testing is initiated.
- If the patient is a child, screen the parents and household members for evidence of infection.
- If resources allow, contact the provider and/or refer the patient to a patient navigator to ensure the patient is receiving care services.
Case Reporting and National Notification
Cases of acute and chronic hepatitis B are nationally notifiable to CDC using a condition-specific event code (Table 1-2). Cases can be re-classified or removed after the initial transmission to CDC if changes are made before surveillance data are finalized each year.
A confirmed acute hepatitis B case may be additionally enumerated as a new confirmed chronic hepatitis B case if a positive HBV detection test (HBsAg, HBeAg, or HBV DNA) is reported 6 months or longer after acute case onset, or if asymptomatic, after the initial positive test result. A case initially classified as confirmed or probable chronic hepatitis B that is determined to be an acute hepatitis B case during the same reporting year should be corrected and reclassified as a confirmed or probable acute hepatitis B case.
A probable acute hepatitis B case that confirms within the same reporting year (before the NNDSS close-out date) can be sent as an update to the same case, but if the case confirms following the initial reporting year, it should not be reported to NNDSS again. A probable chronic hepatitis B case that confirms within the same reporting year (before the NNDSS close-out date) can be sent as an update to the same case, but if the case confirms following the initial reporting year, it should not be reported to NNDSS again. If a case is determined to meet the acute or chronic probable case classification in one reporting year, if additional data are received in a following reporting year that would confirm the case, it should not be reported again to NNDSS.
All confirmed and probable cases are recommended to be transmitted to NNDSS for inclusion in CDC case counts in print criteria. Event conditions for a person should be linked in NNDSS using the same patient ID if submitting via HL7 messages or NBS. See Section 5.2 for additional guidance on transmitting multiple viral hepatitis events for the same person. See Section 3.5 for more information on hepatitis B case reporting and national notification.
Surveillance Activities for Chronic Hepatitis B
The following section provides best practice models for core and enhanced surveillance activities that jurisdictions should consider. Enhanced surveillance activities should be defined based on local priorities.
Best Practice Models For Core and Enhanced Chronic Hepatitis B Surveillance
Core Surveillance
Ascertainment and Reporting
- Create or maintain an electronic system for systematically collecting and storing hepatitis B laboratory results and other case data (e.g., demographic, risk, and clinical information) longitudinally for unique (deduplicated) persons.
- Establish or maintain a method to receive hepatitis B laboratory data and enter it into the hepatitis B surveillance system or registry, preferably through an automated ELR system. ELR is the most efficient way to receive these data, especially if the ELR system can automatically enter the hepatitis B records into the surveillance system.
-
- If an ELR system for other conditions is used in the jurisdiction, include hepatitis B.
- If ELR is not possible, work with high volume testers to receive data in another way (e.g., periodic flat files).
- Implement a process to review and classify cases within the surveillance system or registry.
- Extract data from the hepatitis B surveillance system or registry and transmit cases to CDC according to NNDSS procedures.
-
- Include extended data elements in addition to core data elements, when feasible.
Investigations
- Document local procedures for investigations, including defining priority populations, or identifying priority reports for investigation. See Section 3.2 for types of priority cases.
- Conduct investigations for priority reports or populations. See Section 3.6.5 for chronic hepatitis B case investigation guidance.
- Establish a protocol for identifying and investigating health care-associated infections or coordinate with the department’s health care-associated infections program. Use CDC’s health care-associated infection toolkit as a resource.
- Establish a protocol for identifying and investigating other unique exposures.
Quality Assurance
- Identify and review potential duplicate reports so that only the initial report of each chronic hepatitis B case is counted, and subsequent reports can be used for confirming cases or longer-term monitoring.
- Establish a process for cleaning, reviewing, and standardizing case data and test results.
- Assess case reports and test results for completeness and accuracy.
Analyses
- Create an annual report, situational analysis, or other data product that can be widely shared with providers, advocates, and other public health professionals.
Policy
- Research existing health code/policy related to hepatitis B reporting and the process for changing such policies (if necessary).
- Identify who should report hepatitis B cases – health care providers, health care facilities, and/or laboratories.
- Determine what should be reportable. At a minimum, positive HBsAg, HBeAg, anti-HBc IgM, and HBV DNA results (including genotype) should be reportable. If possible, pregnancy status and concurrent ALT and total bilirubin results should be reported with positive hepatitis B laboratory results, and negative HBsAg and undetectable HBV DNA results should also be reportable. Jurisdictions that receive total anti-HBc laboratory results can use these results to clarify a person’s HBV infection status and confirm chronic cases in conjunction with evidence of positive tests for HBsAg or HBeAg.
- Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate.
Other Data Sources
- Explore how to obtain access to additional sources of data (e.g., vital statistics person-level data).
Enhanced Surveillance (where resources permit)
Ascertainment and Reporting
- Use additional data sources to identify cases not reported to the hepatitis B surveillance system or registry (e.g., vital records and medical records review). See Section 5.4 for a description of optional data sources.
- Use additional data sources to supplement data in the surveillance system or registry. For example:
- Conduct vital statistics death registry matches to update vital status and death date
- Conduct vital statistics birth registry matches to update pregnancy information, and to link gestational parent-infant pairs within the surveillance system in coordination with perinatal hepatitis B prevention program
- Conduct data linkage matches to other disease registries (e.g., HIV, cancer) to find missing information (e.g., race, ethnicity, co-infection, co-morbidities)
- Use a medical record extraction system to identify additional cases and pregnancy status that might not otherwise be reported or to improve efficiency of those reports
- Implement a process for updating cases in the surveillance system or registry with potential health care systems data to track patients along the care continuum (e.g., insurance and pharmacy claims data, hospital discharge data).
Investigations
- Conduct chronic hepatitis B case investigations for additional priority populations. See Section 3.6.5 for chronic hepatitis B case investigation guidance.
- Establish a protocol for identifying and investigating other unique exposures, including clusters or outbreaks.
- Establish methods for identifying reactivations (i.e., determine whether the patient has a history of acute or chronic hepatitis B) to distinguish a new case of disease from reports or notifications that should not be enumerated as a new case.
- If personnel and other resources allow, consider in-depth investigation of a random sample of chronic cases to evaluate demographic variables, reason for testing, access and barriers to prevention and treatment services, and other questions of importance for viral hepatitis elimination activities in the jurisdiction. Personnel with expertise in study design, data collection, and analytic skills should develop and oversee these types of in-depth investigations.
- Assure linkage to care, treatment, and harm reduction services for priority populations where resources allow.
Quality Assurance
- Establish additional quality assurance processes for case reports and test results.
- Implement quality assurance improvement measures to ensure completeness and accuracy of case investigations and interpretation of laboratory results.
- Establish systems to identify and address decreases in laboratory reporting by test type volume and laboratory that might represent coding or transmission issues.
- Establish systems to identify and address deficiencies in provider reporting (e.g., incomplete or missing reports).
Analyses
- Use data linkage matches to other disease databases/registries (e.g., HIV, HCV, and cancer) for analysis of co-infections and identification of receipt of care.
- Use vital statistics birth registry matches for analysis of infants born to HBV-infected gestational parents.
- Use death registry matches to describe hepatitis B-associated mortality.
- Create provider-level indicators (such as complete reporting, complete diagnostic testing, linkage to care, and treatment initiation) to work with providers to improve these outcomes.
- Identify methods for establishing surveillance-based chronic hepatitis B prevalence estimates.
- Identify and describe trends and disparities in liver cancer incidence and mortality.
- Create hepatitis B care continua, including determining and validating surveillance-based definitions for hepatitis B treatment and outcome indicators.
- Identify and describe trends and disparities along the care continuum (e.g., disparities in screening, viremia, linkage to care, and treatment initiation).
- Expand the data reports available to external partners.
Policy
- Use surveillance data and best practices from other jurisdictions to recommend health code changes related to reporting (e.g., obtaining non-positive test results), as allowable within the jurisdiction.
- Use surveillance data to support evidence-based for health code changes related to expanding access to syringe services programs and other harm reduction services for populations affected by hepatitis B, as allowable within the jurisdiction.
- Use analysis of surveillance data on trends and disparities to guide resource allocation and inform public health action, prioritizing those communities most disproportionately affected.
Other Data Sources
- Obtain access to supplemental data sources wherever possible and incorporate their usage into routine practices. See Section 5.4 for a description of optional data sources.
Considerations for Hepatitis B Cases who were Transplant Recipients
Organ and tissue donor-derived HBV infection is rare and commonly associated with IDU in a deceased donor (66). In the 2020 Public Health Service (PHS) guidelines (67), it is recommended that all organ recipients in the United States receive hepatitis B vaccination, pre-transplant testing for total anti-HBc, HBsAg and anti-HBs, and post-transplant testing for HBV DNA at 4–6 weeks.
However, clinical manifestations of post-transplant HBV infection can be delayed by many months after liver transplantation (68, 69). As such, the 2020 PHS guidelines also recommend that health care providers caring for liver recipients consider conducting additional testing by HBV NAT or assessing signs or symptoms of liver injury (e.g., jaundice or elevated liver function tests) at one-year post-transplant. All donors are screened for total anti-HBc, HBsAg, and HBV DNA prior to organ procurement. In situations where the donor is known to be positive for any of these tests, recipient HBV infection is expected and does not require investigation by the HD beyond notifying CDC that the recipient case is donor derived. As these patients are already linked to testing and treatment, these infections are notifiable to CDC as new acute cases, but the jurisdiction need not investigate beyond indicating that the infection was donor-derived.
Organ transplantation from deceased donors dying of overdose and IDU has increased recently (70). To facilitate identification of suspected donor-derived cases of viral hepatitis, jurisdictional viral hepatitis surveillance programs should consider reaching out to transplant centers proactively. In most jurisdictions, there are a relatively small number of medical centers that perform transplantation (71). A listing of transplant facilities in the United States, including facility location and phone number, can be found on the Organ Procurement and Transplantation Network (OPTN) website (71).
Knowing whether the transplant center is using organs from deceased donors who injected drugs or who were positive for anti-HCV or HCV RNA is important, as this might increase the very small risk of donor-derived HBV infection (69). When donor-derived viral hepatitis is suspected, the transplant center is required to report the infection to the Disease Transmission Advisory Committee (DTAC) of OPTN, which often consults with CDC about a possible investigation. If CDC accepts the investigation, it is coordinated by the CDC Office of Blood, Organ, and Other Tissue Safety with consultation from CDC DVH. CDC only investigates selected reports of “unexpected” viral hepatitis transmission, meaning that both the donor and recipient tested negative for hepatitis B (including anti-HBc, HBsAg and HBV DNA, if available) prior to the transplant.
Investigation includes review of all laboratory and clinical data for donor and recipients and testing archived donor samples (e.g., serum, lymphocytes, and liver biopsy), if available, for HBV DNA. When the initial investigation is complete, CDC DVH contacts the public health jurisdiction to complete the rest of the investigation. Typically, there are two outstanding questions that only the public health jurisdiction can answer: 1) Did the recipient have any behavioral or other risks for hepatitis B (e.g., IDU) and 2) Does the jurisdiction have any ongoing investigations of health care-associated hepatitis B that might be related to this investigation?
Case classification in patients with a documented transplant should consider reports of laboratory test results prior to and post-transplant and potential health care exposures, if suspected. Table 3-4 outlines considerations for hepatitis B cases who were transplant recipients of a solid organ.
Table 3-4. Considerations for hepatitis B cases who received a solid organ from a donor*
| Organ Recipient Pre-transplant
Laboratory Result† |
Organ Recipient Post-transplant
Laboratory Result† |
Case Classification |
|---|---|---|
| Positive hepatitis B surface antigen (HBsAg), hepatitis B ‘e’ antigen (HBeAg), or HBV DNA | Positive HBsAg, HBeAg, or HBV DNA | Should not be considered a new case due to organ transplant, but rather an infection documented prior to transplant. To determine whether this case should be considered newly reported, follow Figure 3-3. |
Evidence of resolved prior infection:
|
Evidence of reactivation:
|
Should not be considered a new case, but reactivation of prior infection. Reactivation information should be appended to the case record of the existing case in the jurisdiction’s surveillance system. |
| Negative HBsAg
Negative total anti-HBc |
Positive HBsAg, HBeAg, or HBV
DNA |
Three major potential possibilities should be considered:
Centers for Disease Control and Prevention (CDC)’s Division of Viral Hepatitis (DVH) might already have been notified and is available for consultation and coordination of investigation. |
| No prior HBV laboratory results‡ |
*All donors should be tested for total anti-HBc, HBsAg and HBV DNA prior to organ procurement (63). This table applies to recipients of organs from donors who tested negative for all these markers.
†Because of the large number of tests performed on transplant recipients, irreproducible positive results are rarely reported. Investigators should evaluate all available results in context. CDC DVH is available for consultation.
‡Pre-transplant hepatitis B screening (total anti-HBc, HBsAg and anti-HBs) is recommended for all transplant recipient candidates in accordance with guidelines published by the US Public Health Service (63). If a transplant recipient does not have hepatitis B laboratory results prior to transplantation of an organ, consider following-up with the transplant facility to discuss appropriate pre-transplant hepatitis B screening protocols.
Download of this table: PDF
Cases of viral hepatitis identified among living organ transplant donors and recipients should be submitted to NNDSS in a standardized way, when possible. The CDC case report forms used for NBS and HL7 transmission both include a reason for testing variable in the core section of the form. For HDs transmitting data via NBS or HL7, under the “reason for testing” field, “blood/organ donor screening” should be selected for organ donor cases; “other” should be selected for organ transplant recipient cases with a specification of “transplant recipient” in the free text for the “other reason for testing” field. For state and territorial HDs transmitting case data via NETSS, there is no field on the case report form to indicate that the case was an organ transplant donor or recipient.
Monitoring Infection Trends and Disease Outcomes Using a Person-Level Database and Supplemental Data Sources
A person-level surveillance database can support hepatitis B elimination efforts by allowing a jurisdiction to document hepatitis B laboratory results and testing history. By doing so, jurisdictions are able to:
- track the number of unique persons living with hepatitis B longitudinally, which can inform more accurate estimates of incidence and prevalence;
- identify pregnant people and infants for the PHBPP;
- identify and link people living with hepatitis B to medical care;
- evaluate the impact of public health and clinical services;
- match with secondary data sources (e.g., Vital Statistics, Medicaid, cancer registry, HIV registries); and
- provide information on the number of people at each phase of the hepatitis B care continuum to identify areas for improvement, for example, by supplementing surveillance data with clinical and pharmacy data.
Some of these monitoring capacities may only be possible in jurisdictions capable of capturing negative hepatitis B laboratory results. Jurisdictions are encouraged to require reporting of negative/undetectable HBV DNA results as these results can be a proxy indicator that people living with hepatitis B have been linked to care. This information can support accurate longitudinal surveillance and measurement of hepatitis B care continua. Linking a person-level surveillance database to other data sources not only allows for longitudinal monitoring of disease outcomes, but can also improve completeness of information in the surveillance system (68). Supplemental data sources are helpful for understanding the burden of co-morbidities (e.g., infection with HCV or HIV) by providing cross-sectional data over time and can inform interpretation of prevalence estimates. Section 5.4. describes supplemental data sources for HDs to consider.
Surveillance of Hepatitis B During Pregnancy and Perinatal Hepatitis B
Uses of Surveillance Data
Surveillance Case Definition
Case Ascertainment
Case Investigation
Case Management
Case Reporting and National Notification
Background
Knowledge of a pregnant person’s HBV infection status is essential for preventing perinatal hepatitis B. The American College of Obstetrics and Gynecologists (ACOG) supports CDC’s recommendation that prenatal care providers should screen every pregnant person for HBV infection during an early prenatal visit, even if the person has already been vaccinated or tested for hepatitis B (73). HBV particles have also been detected in ova (74, 75); though uncommon, the potential to vertically transmit HBV exists when an HBV-infected genetic parent donating ova elects to use a gestational carrier (i.e., surrogate). Transmission of HBV infection at birth leads to chronic infection in approximately 90% of infants who are not given immunoprophylaxis (76).
To improve the prevention and identification of perinatal hepatitis B and to facilitate the clinical care of pregnant and postpartum people, universal HBsAg screening during an early prenatal visit and treatment of infants born to HBsAg-positive gestational parents with hepatitis B immunoglobulin and hepatitis B vaccine at birth were recommended in 1988 by ACIP (73). To reduce perinatal transmission risks, it is recommended that pregnant people with an HBV DNA level >200,000 IU/mL receive antiviral therapy at 28–32 weeks of gestation and infants born to HBV-infected gestational parents receive HBIG at birth (50, 73). However, it is estimated that approximately 1,000 newborns are infected annually despite these longstanding recommendations (77).
Surveillance should include monitoring HBV-infected pregnant people and monitoring infants born to them for receipt of immunoprophylaxis at birth, completion of the infant hepatitis B vaccination series, and PVST for evidence of infection (HBsAg-positivity or HBV DNA-positivity) and immunity (anti-HBs >10 mIU/mL). PVST identifies infants who failed to respond to the hepatitis B vaccine and require re-vaccination.
The overall surveillance goals of hepatitis B during pregnancy include: 1) identifying pregnant people currently infected with HBV (as indicated by the presence of HBsAg or HBV DNA), and 2) among HBV-infected people of childbearing age with childbearing potential, identifying those who are currently pregnant or who have recently delivered a live birth to identify exposed infants for referral to the PHBPP.
Perinatal hepatitis B surveillance relies on screening for HBsAg during each pregnancy and conducting the appropriate follow-up tests on infants born to HBV-infected gestational parents. The overall goals of perinatal hepatitis B surveillance are to identify exposed infants and evaluate the effectiveness of the PHBPP to prevent perinatal transmission, and also the following:
- identify HBV-infected people of childbearing age with childbearing potential to link them to care to prevent infant HBV exposure during future pregnancies;
- provide data to improve assessment of the burden of perinatal hepatitis B;
- evaluate health outcomes of HBV-infected infants;
- educate clinicians and guardians on HBV transmission, clinical progression, and treatment; and
- measure the rate of progression to chronic hepatitis B, as determined by HBsAg-positivity or by the detection of HBV DNA after 24 months of age.
Uses of Surveillance Data
Surveillance data are used in the following ways to accomplish the above goals:
- Identifying HBV-infected pregnant people to prevent perinatal HBV transmission.
- Monitoring and evaluating the effectiveness of PHBPP.*
The following key indicators are used for pregnant people:- Number of HBV-infected pregnant people identified
- Number of births to HBV-infected pregnant people identified
The following key indicators are used for exposed infants:
- Number and percentage of exposed infants who receive first dose of hepatitis B vaccine and HBIG within 12 hours of birth.
- Number and percentage of exposed infants who receive HBIG and complete the hepatitis B vaccine series by 6 months of age.
- Number and percentage of exposed infants who receive PVST consisting of HBsAg and anti-HBs:
- Infants at 9–24 months of age
- Number who tested positive for HBsAg
- Number with anti-HBs titer level of <10 mIU/mL
- Children at >24 months of age
- Number who test positive for HBsAg (reported as chronic hepatitis B)
- Infants at 0–24 months of age
- Number of erroneous tests performed (e.g., testing performed outside of the recommended age windows or wrong test ordered)
- Infants at 9–24 months of age
- Number of exposed infants who require additional doses of vaccine (non-responders)
- Number of exposed infants lost to follow-up
- Assessing the frequency and evaluating the causes of missed opportunities. This includes evaluating missed opportunities for testing during pregnancy and for antiviral therapy, when indicated. For HBV-exposed infants, this includes failure to provide timely immunoprophylaxis and vaccination failure. It is recommended that all exposed infants receive the first dose of the hepatitis B vaccine and HBIG within 12 hours of birth, even those with low birth weight (i.e., birth weight <2,000 g). Investigation of perinatal hepatitis B cases should evaluate causes of possible breakthrough infections and should include obtaining sera from the infant and gestational parent to test for the presence of HBV variants.
- Monitoring adherence to screening recommendations among pregnant people.Surveillance programs should ideally collect negative HBV laboratory results. Alternatively, surveillance can help track changes in hepatitis B incidence and be used to implement quality measures to monitor adherence to screening recommendations.
- Monitoring trends in disease incidence and prevalence among people of childbearing age with childbearing potential. Knowing the incidence and prevalence of hepatitis B in the population who are or can become pregnant is critical to the prevention and control of hepatitis B, and this population should be assessed independently from surveillance in the general population.
*Note that the outcome indicators for PHBPP are slightly different from the ACIP recommendations (56).
Surveillance Case Definition
No CDC/CSTE surveillance case definition exists for HBV infection during pregnancy. Instead, these cases should be classified in accordance with the CDC/CSTE acute and chronic hepatitis B case definitions (see Section 3.6.3).
Table 3-5 depicts the surveillance case definition for perinatal hepatitis B, adopted by CSTE and CDC in 2017 (63, 64). See Appendix C for classification scenarios of cases of perinatal hepatitis B.
Table 3-5. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for perinatal hepatitis B, 2017
| Criteria Type | Ctriteria |
|---|---|
| Demographic |
|
| Clinical |
|
| Laboratory* | Child <24 months of age with evidence of hepatitis B as shown by the following laboratory results:
|
| Epidemiologic Linkage |
|
| Case Status | Classification |
| Confirmed Perinatal* |
|
| Probable Perinatal* |
|
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to care, as appropriate.
†Positive HBsAg results obtained from infants ≤9 months of age who received hepatitis B vaccine should not be interpreted as positive due to the potential for transient HBsAg positivity.
Downloads of this table: PDF | PPT
HBsAg test results obtained from infants ≤1 month of age, and HBeAg and HBV DNA results from those ≤9 months of age should not be used for classification. Cases in the specified age range that are known to have been exposed to HBV through health care and not perinatally should also be classified under the 2012 acute and chronic hepatitis B case definition. The event date of the perinatal hepatitis B case should be based on the earliest relevant laboratory test collection date within the test-specific age window.
Case Ascertainment
To facilitate identification of HBV infection during pregnancy, the following measures are recommended:
- Screen for HBsAg during each pregnancy as part of prenatal care at an early prenatal visit (i.e., during the first trimester)
- For pregnant people who are isolated total anti-HBc positive, check HBV DNA status to determine if occult HBV infection or HBsAg mutant infection is present
- Document maternal HBV infection status on newborn metabolic screening card or birth certificate
- Report laboratory results indicating HBV infection for all pregnant people to the appropriate health jurisdiction
- Ensure delivery facilities have standing orders to
- Check HBV infection status upon admission. The following groups should be tested for HBsAg at the time of admission for delivery (56):
- Those whose HBsAg status is unknown
- Those with clinical hepatitis
- Those with high risk behaviors (e.g., history of recent or current IDU, multiple sexual partners within the past 6 months or an HBsAg-positive sexual partner, or evaluation or treatment for a sexually transmitted infection)
- Check HBV infection status upon admission. The following groups should be tested for HBsAg at the time of admission for delivery (56):
- Report admission of HBV-infected pregnant people to delivery facility
- Ensure health care providers have protocols to:
- Test for HBV infection status during each pregnancy at an early prenatal visit (i.e., during the first trimester)
- Ensure that state, territorial, and local health jurisdictions can:
- Receive and integrate electronic laboratory reports, electronic health records, and facsimiles into a disease surveillance system
- Perform enhanced surveillance methods to identify previously unreported HBV-infected people who have recently given birth by comparing birth certificate data to known HBV-infected cases in the disease surveillance system
- Determine pregnancy status of all HBsAg-positive people of childbearing age with childbearing potential
- Determine pregnancy status for all existing cases of hepatitis B among people of childbearing age with childbearing potential
The following steps are recommended to facilitate identification of perinatally HBV-exposed infants:
- Ensure delivery facilities have standing orders to report all births to HBsAg-positive or HBV DNA-positive gestational parents to local PHBPP jurisdiction
- Ensure health care providers have protocols to:
- Test all exposed infants for HBsAg and anti-HBs (PVST) at 9–12 months of age
- Routinely report the hepatitis B PVST results of exposed infants to the local health jurisdiction
Figure 3-4 illustrates a potential approach for perinatal hepatitis B case ascertainment and classification. Specific procedures vary by jurisdiction, but should generally follow the scheme below, in accordance with the CDC/CSTE Position Statement for the 2017 perinatal hepatitis B case definition (63, 64).
Figure 3-4. Process for perinatal hepatitis B case ascertainment and classification
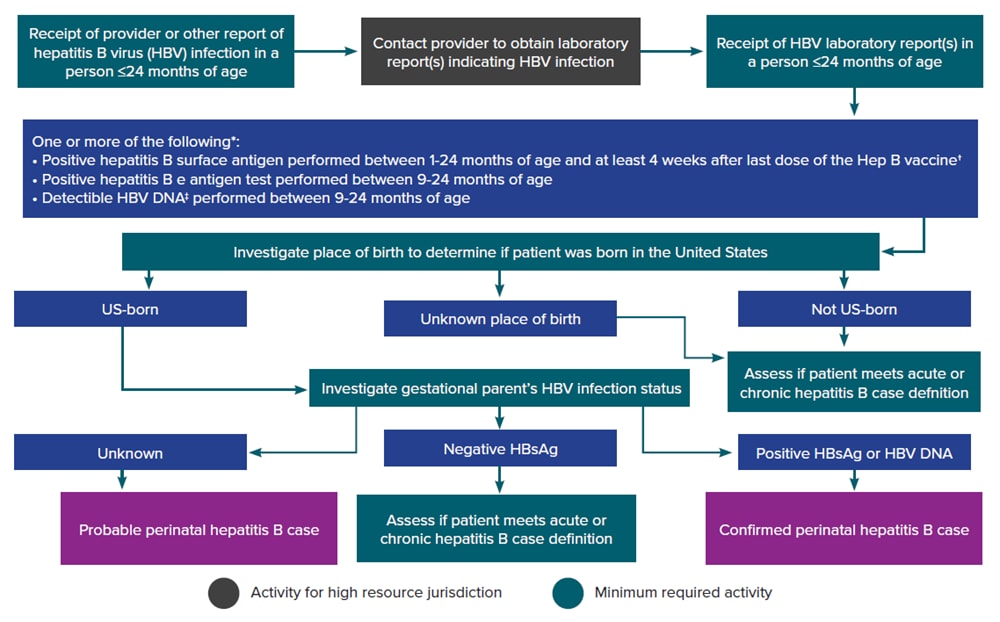
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to care, as appropriate. HBsAg test results obtained from infants ≤1 month of age and hepatitis B e antigen and HBV DNA results obtained from those ≤9 months of age should not be used for classification. Cases among children <24 months of age who are known to have been exposed to HBV through health care (not perinatally) should be reported according to the 2012 acute and chronic hepatitis B case definitions.
†Positive HBsAg results obtained from infants ≤9 months of age who received hepatitis B vaccine should not be interpreted as positive due to the potential for transient HBsAg positivity.
‡Nucleic acid testing for HBV DNA, including qualitative, quantitative, and genotype testing.
Downloads of this figure: PDF | PPT
Case Investigation
The following elements can inform investigation and management of HBV-infected pregnant people and infants:
- Demographic information. For the pregnant person, obtain date of birth, current gender, race, ethnicity, residential address (including zip code), insurance status, country of birth, and primary language spoken. For the infant, obtain the date, time, and place of birth, birth weight, sex, race, ethnicity, and insurance status. The contact information of the legal guardian(s) should also be collected.
- Patient and health care provider information. Includes prenatal care provider’s name and phone number to coordinate follow-up HBV DNA testing and treatment, if indicated. The contact information of the infant’s health care provider (to obtain PVST results) and legal guardian(s) as well as adoption or foster care status should also be collected.
- Delivery information. Includes the expected and actual due dates and the expected and actual delivery facilities.
- Diagnostic test results. Obtain documentation of positive HBV test results for both gestational parent and infant; obtain anti-HBs test result(s) for the infant.
- Clinical features. For the pregnant person, document the presence of symptoms and jaundice. Most infants with hepatitis B are asymptomatic. Obtain documentation of the gestational parent’s HBV DNA level and whether antiviral medication was administered during 28–32 weeks of gestation.
- Immunization and prophylaxis history. Ascertain the date and time for all administered doses of hepatitis B vaccine and HBIG.
- Epidemiologic link. For the infant, confirm birth to an HBV-infected gestational parent.
- Reporting information. Date reported to health jurisdiction, date of diagnosis, date investigation initiated, date of first contact with patient and/or health care provider, and date referred for medical evaluation.
- Education and referral for follow-up. Provide education to the patient/provider regarding the role of PHBPP, importance of immunoprophylaxis for the infant within 12 hours of birth, and timely completion of the hepatitis B vaccine series and PVST. Determine medical care provider for HBV-infected pregnant cases and educate on the importance of regular care and monitoring of hepatitis B, even after delivery.
Case Investigation Prioritization
The following factors should be considered of high priority for investigation and follow-up:
- Investigation and follow-up should occur during pregnancy or as soon as possible thereafter. Successful follow-up might be more likely if contact information is utilized sooner. Early identification and timely follow-up will facilitate the prevention of perinatal HBV transmission.
- Investigation and follow-up should occur for those who can become pregnant and those whose pregnancy status is unknown who are co-infected with HIV/HCV/STIs or who have high HBV DNA levels (> 200,000 IU/mL).
Case Management
HBV-infected gestational parents and their infant(s) should be tracked in a surveillance database/system that can track case management processes and allow for sharing and/or linking parent and child events. The system should allow for each pregnancy to be considered unique and the pregnancy-specific data to be captured and maintained. It should also contain a mechanism to track hepatitis B vaccine doses given to the infant to ensure the proper number and spacing of doses.
HBV-infected pregnant people should be enrolled in the PHBPP. Upon enrollment, the hepatitis B coordinator should undertake the following case-management actions:
- Obtain a copy of the original laboratory results to provide to the anticipated delivery facility and infant’s anticipated health care provider. Original laboratory reports are strongly recommended to prevent misinterpretation or transcription errors.
- Follow-up with the infant’s anticipated health care provider.
- Notify of maternal HBV infection status (i.e., positive HBsAg or HBV DNA) and communicate the need for timely vaccination and PVST.
- Provide instructions on how to report each hepatitis B vaccine dose administered and the HBsAg and anti-HBs results at 9–12 months of age.
- For low birth weight babies (i.e., those <2,000 g), the initial dose of hepatitis B vaccine should still be administered as early as possible, but should not be counted as part of the vaccine series. The infant should receive 3 additional doses of the vaccine, typically starting at 1 month of age.
- Send a reminder (preferably a phone call) regarding the need for PVST after the final dose of hepatitis B vaccine is administered.
- PVST should include HBsAg testing to ensure the infant has not become infected and anti-HBs testing to ensure that immunity has been conferred (i.e., anti-HBs >10 mIU/mL). Table 3-7 lists the most common laboratory codes for PVST.
- Ensure that susceptible children (i.e., anti-HBs <10 mIU/mL performed >4 weeks after the last dose of hepatitis B vaccine) receive a booster dose and are retested to confirm immunity.
- If the child’s anti-HBs titer is still <10 mIU/mL, complete the second revaccination series and retest for HBsAg and anti-HBs.
- If the anti-HBs titer is still <10 mIU/mL after full vaccination series, the child is considered a vaccine non-responder. Provide guidance to the parent(s) or legal guardian(s) of the child that remains susceptible. For more detailed guidance, see the Epidemiology and Prevention of Vaccine-Preventable Diseases from The Pink Book.
- Refer for HBV DNA testing to determine if antiviral treatment is recommended.
- It is recommended that pregnant people with an HBV DNA level >200,000 IU/mL start antiviral therapy at 28–32 weeks of gestation to reduce perinatal transmission risks (50).
- Document the gestational parent’s HBV DNA level at the time of delivery and whether antiviral medication was received during pregnancy.
- Obtain information on anticipated due date, anticipated delivery facility, actual delivery date, and actual delivery facility.
- Ensure anticipated birth facility is aware of the pregnant person’s HBV infection status and anticipated due date and will report to local jurisdiction’s PHBPP upon delivery of infant.
- 2–4 weeks prior to anticipated due date, send communication to:
- Parent or provider to confirm anticipated delivery facility
- Anticipated delivery facility to communicate the need to
- provide timely post-exposure prophylaxis for infant,
- report birth and prophylaxis information to public health, and
- administer the first dose of the hepatitis B vaccine and HBIG in separate anatomical sites.
- Document the first dose of hepatitis B vaccine given within 12 hours of birth.
- Document completion of hepatitis B vaccine series in accordance with ACIP recommendations.
- Refer children with positive HBsAg test results for evaluation (by referral or consultation, if appropriate), ensuring
- confirmation of chronic hepatitis B (tests positive for HBsAg after 24 months), as spontaneous clearance is possible;
- assessment of evidence of chronic liver disease; and
- assessment of severity of disease and possible treatment according to current practice guidelines in consultation with, or by referral to, a specialist knowledgeable in this area.
- All parents should be provided with education regarding HBV vertical transmission and testing recommendations. Providers should also be provided with information on HBV transmission risk to the infant, hepatitis B testing recommendations (PVST), and HBV infection clinical care. Education should be provided according to evidence-based guidelines and include perinatal transmission risk, testing for the infant, and current treatment recommendations.
The following case management actions are recommended for delivery facilities:
- Report gestational parent’s HBV infection status (i.e., positive HBsAg or HBV DNA) on infant’s birth certificate and metabolic screening card (as applicable)
- Report birth to an HBV-infected gestational parent to appropriate health jurisdiction. This can be achieved by completing the jurisdiction-specific reporting form with all requested information
- Facilities that need information regarding immunoprophylaxis for infants born to HBV-infected gestational parents can contact the Perinatal Hepatitis B Prevention Program in their state or territory.
Case management is considered completed when the
- child has evidence of immunity,
- child has moved to another jurisdiction and notification to that jurisdiction and PHBPP has been completed, or
- gestational parent-infant pair is lost to follow-up.
Sources of data on immunization and PVST include, but are not limited to
- jurisdiction’s immunization registry/information system,
- report from infant’s health care provider, and
- electronic health and laboratory records.
Table 3-6. Common laboratory codes for hepatitis B post-vaccination serologic testing
| Laboratory | Hepatitis B Surface Antigen | Antibody to Hepatitis B Surface Antigen |
|---|---|---|
| Affiliated Medical Services | 5196-1 | 10900-9 |
| LabCorp | 006510 | 006530 |
| Mayo Medical Labs | 9013 | 8254 |
| Quest Diagnostics | 498 | 8475 |
Downloads of this table: PDF | PPT
Case Reporting and National Notification
HBV infection during pregnancy should be a reportable event to the HD. Although no specific NNDSS event exists for hepatitis B during pregnancy, CDC recommends classifying these cases as acute or chronic using the appropriate event code (Table 1-2) in accordance with the CDC/CSTE case definitions, and transmitting the pregnancy status to NNDSS for all cases among people of childbearing age with childbearing potential to allow for national tracking. At the jurisdiction-level, there may be classification category specifically for hepatitis B during pregnancy. Cases of perinatal hepatitis B are nationally notifiable to CDC and are submitted using a condition-specific event code (Table 1-2). A case classified as perinatal hepatitis B can be additionally enumerated as a confirmed case of chronic hepatitis B if a positive HBV viral detection test (HBsAg, HBeAg, or HBV DNA) is obtained after the case is >24 months of age.