Appendices
Updated February 29, 2024
Appendix A. Glossary
42 Code of Federal Regulations (CFR) Part 2: A federal regulation that protects the confidentiality of substance use disorder client records with no exemption for public health access without specific consent.
Acute viral hepatitis: The early stage of a viral infection of the liver caused by one of five different hepatitis viruses (A, B, C, D, or E). Signs and symptoms of early (or acute) viral hepatitis include yellowing of the skin or eyes (jaundice), abdominal pain, vomiting, nausea, diarrhea, malaise, grey-colored stools, and dark urine. For hepatitis B, hepatitis C, hepatitis D, and hepatitis E, acute infection can lead to chronic infection.
Case status: The classification of the condition utilizing the Centers for Disease Control and Prevention (CDC)/Council of State and Territorial Epidemiologists (CSTE) viral hepatitis case definitions (i.e., confirmed, probable, and not a case).
Chronic viral hepatitis: A long-term illness that occurs when a hepatitis virus infection persists. Chronic hepatitis can last a lifetime and lead to serious liver problems, including liver cirrhosis and liver cancer.
Coinfection: Simultaneous infection with two or more separate pathogens.
Condition: In regard to viral hepatitis, the type of infection and situation (e.g., hepatitis A, acute hepatitis B, chronic hepatitis B, perinatal hepatitis B, hepatitis B during pregnancy, acute hepatitis C, chronic hepatitis C, perinatal hepatitis C, and hepatitis C during pregnancy).
Event: A paper or electronic laboratory report, or an occurrence of disease or reportable condition.
Event code: A standardized, unique code that is assigned to each disease or condition to simplify storage and retrieval of information about cases transmitted to the National Notifiable Diseases Surveillance System (NNDSS).
Event date: A variable in the National Notifiable Diseases Surveillance System generated by the Office of Public Health Data, Surveillance, and Technology that is based on a hierarchy of dates. In decreasing order of specificity, these are date of disease/symptom onset, date of specimen collection/diagnosis, date of laboratory result receipt, date of first report to health department, and state/territory or MMWR report date.
Field-based or community-based staff: Terms used to describe health department staff (such as disease intervention specialists or DIS) who conduct patient and provider interviews.
HBV-infected: People who are positive for hepatitis B surface antigen (HBsAg) and/or hepatitis B virus deoxyribonucleic acid (HBV DNA).
HCV detection test: A nucleic acid test (NAT) for hepatitis C virus (HCV) ribonucleic acid (RNA) may be qualitative, quantitative, or done for genotype testing, or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). When these tests become available, they will serve as acceptable laboratory criteria for active infection, equivalent to HCV RNA testing.
HCV-positive: People who are either: 1) positive for HCV RNA; or 2) positive for HCV antibody with no evidence of an HCV RNA test being conducted. Until the HCV RNA status is known, out of caution, cases should be considered HCV-positive for perinatal HCV exposures and pregnant people.
Hepatitis A: A vaccine-preventable, communicable disease of the liver caused by the hepatitis A virus (HAV) and usually transmitted person-to-person through the fecal-oral route or consumption of contaminated food or water. Hepatitis A is a self-limited disease that does not result in chronic infection.
Hepatitis B: A vaccine-preventable, communicable disease of the liver caused by HBV and transmitted when blood, semen, or another body fluid from an infected person enters the body of someone who is not infected, including from parent to child at birth. Hepatitis B can be a short-term illness, but for others, it can become a long-term, chronic infection. The likelihood of chronic infection is inversely associated with age at infection.
Hepatitis C: A communicable disease of the liver caused by HCV transmitted when blood from an infected person enters the body of someone who is not infected, including from parent to child at birth. Hepatitis C can be a short-term illness, but for approximately three fourths of people who become infected, it can become a long-term, chronic infection.
HIPAA Privacy Rule 45 CFR 164.512(b): A federal privacy rule that allows public health authorities and others responsible for protecting the public’s health and safety to collect and receive protected health information without patient authorization for the purpose of preventing and/or controlling disease, injury or disability, including the conducting of public health surveillance, public health investigations, and public health interventions.
Investigation start date: The date a case investigation was opened.
Laboratory report: A paper or electronic laboratory report entered within a jurisdiction’s surveillance system.
Medication assisted treatment (MAT): Medication assisted treatment is the use of opioid substitution medication for opioid use disorder (MOUD) in conjunction with a variety of behavioral support interventions. See also medication for opioid use disorder.
Medication for opioid use disorder (MOUD): Any licensed medication such as methadone, buprenorphine or naltrexone used for treatment of opioid use disorder. For information on MOUD, visit the Substance Abuse and Mental Health Services Administration (SAMHSA) and the Providers Clinical Support System websites.
NNDSS Modernization Initiative: A multi-year initiative under the CDC Surveillance Strategy that aims to enhance the ability of NNDSS to provide more comprehensive, timely, and higher quality data for public health decision making. The NNDSS Modernization Initiative involves collaboration with disease-specific programs at CDC and health departments to develop disease-specific data elements for new message mapping guides (MMGs) for Health Level 7 (HL7)-formatted disease case notification. CDC is increasing the robustness of the NNDSS technological infrastructure so that it is based on interoperable, standardized data and exchange mechanisms.
Notifiable: The conditions that CSTE recommends state and territorial health departments perform surveillance upon and notify to CDC.
Opioid use disorder: Substance use disorder involving opioid medications. See substance use disorder.
Pregnant person, person of childbearing age with childbearing potential, and gestational parent: Terminology used to describe a parent who is pregnant, has the potential to become pregnant, or has physically given birth, regardless of gender.
Print criteria: The standards under which CDC can publish case information, as determined by CSTE and CDC and listed in CSTE’s Position Statements.
Reportable: The conditions that are required to be reported to the local, state or territorial health department.
Substance use disorder: A medical condition of addiction in which a person is compelled by physiological dependence on a legal or illegal drug. For more information, review the consumer version of the Merck Manual on substance use disorders.
Surveillance case definition: Criteria defined in CSTE’s Position Statements to provide uniform case ascertainment, case classification, and consistent national notification of nationally notifiable conditions.
Superinfection: A second infection that occurs in addition to an existing infection.
Suspected hepatitis A and/or B vaccine failure: Occurs when a person who has completed the hepatitis A and/or hepatitis B vaccine series according to the appropriate immunization schedule becomes infected >30 days after vaccine series completion.
Sustained virologic response: Occurs when a person’s HCV infection is considered to be cured when HCV RNA is undetectable in the blood at or after 12 weeks following treatment completion.
Syringe services program (SSP): A community-based facility, mobile unit, or other organized program whose mission includes distribution of sterile paraphernalia for injection of drugs and safe disposal of used paraphernalia without stigma or judgement. Paraphernalia includes syringes, needles, cookers and all other needed supplies for injection of drugs. Comprehensive SSPs include not only distribution and safe disposal of injection supplies, but multiple other services needed by people who inject drugs including, but not limited to, naloxone distribution and training; vaccination for hepatitis A and hepatitis B; testing and treatment or linkage to treatment for HBV, HCV, HIV, and sexually transmitted infections; pre-exposure prophylaxis for HIV; treatment or linkage to treatment for substance use disorder; and patient-centered reproductive health care, including access to long-acting reversible contraceptives. For more information, visit the CDC website on SSPs.
Window period: The period of time after a person is infected with a communicable disease but before laboratory eveidence (e.g., antibodies) of infection is detectable on testing. During the window period, a patient’s antibody test will be negative even though the patient is infected.
Appendix B. Description of Hepatitis A, Hepatitis B, and Hepatitis C Laboratory Markers
| Laboratory Marker | Description |
|---|---|
| Hepatitis A | |
| Immunoglobulin M antibody to hepatitis A virus (anti-HAV IgM) | Indicates hepatitis A virus (HAV) infection. On average, appears approximately 5–10 days before the onset of clinical symptoms and can circulate for up to 6 months in the bloodstream following infection. |
| Immunoglobulin G antibody to hepatitis A virus (anti-HAV IgG) | Indicates recovery from HAV infection, past infection, or vaccine-induced immunity. Remains in serum for life-long protection. |
| Total hepatitis A virus antibody (total anti-HAV) | Indicates current HAV infection (if also positive for anti-HAV IgM) or immunity to hepatitis A from past infection or vaccination (negative for anti-HAV IgM). Consists of both IgM and IgG class antibodies. |
| Hepatitis A virus ribonucleic acid (HAV RNA) | Indicates current HAV infection. During an outbreak, people might be tested for the presence of HAV RNA, and if detectable, HAV may be genotyped for specific strain of virus. HAV RNA is the most sensitive and specific indicator of current infection, and if quantified, correlates with levels of HAV in serum or plasma, measured in IU/mL. |
| HAV genotype | Categorizes the specific HAV genetic strain with which a person is infected. When a genotype is determined, it indicates detection of current HAV infection. |
| Hepatitis B | |
| Hepatitis B surface antigen (HBsAg) | Indicates current hepatitis B virus (HBV) infection. Also indicates chronic hepatitis B in those who are positive for HBsAg for >6 months. |
| IgM antibody to hepatitis B core antigen (anti-HBc IgM) | Indicates acute HBV infection. |
| Total hepatitis B core antibody (total anti-HBc) | Indicates current hepatitis B (in someone with a positive HBsAg) or past hepatitis B (in someone with a negative HBsAg). On average, appears approximately 5 weeks post HBV exposure. Consists of both IgM and IgG class antibodies. After approximately 6 months, anti-HBc IgM becomes undetectable, whereas anti-HBc IgG persists indefinitely. |
| Hepatitis B surface antibody (anti-HBs) | Indicates recovery and immunity from hepatitis B. Anti-HBs is also detected in people who develop immunity through hepatitis B vaccination. According to the World Health Organization, anti-HBs levels >10mIU/mL indicate adequate immunity. |
| Hepatitis B virus deoxyribonucleic acid (HBV DNA) | Indicates chronic hepatitis B in those with detectable HBV DNA for >6 months and occult hepatitis B and HBsAg mutant infection in those who test positive but are negative for HBsAg. It is the most sensitive and specific indicator of current infection, and if quantified, correlates with levels of HBV in serum or plasma, measured in IUI/mL. |
| HBV genotype | Categorizes the specific HBV genetic strain with which a person is infected, which can affect the natural history of chronic infection. When a genotype is determined, it indicates current detection of HBV infection. |
| Hepatitis B e antigen (HBeAg) | Indicates current infection. The presence of HBeAg indicates that the virus is replicating, and the infected person is likely to have high levels of HBV DNA. |
| Hepatitis B e antibody (anti-HBe) | Spontaneous conversion from e antigen positivity to e antibody positivity (a change known as “seroconversion”) develops after resolution of acute infection and can occur spontaneously in the evolution of chronic infection. Seroconversion also may happen among HBeAg-positive, chronically infected people after they receive treatment. This marker is not used in hepatitis B surveillance case classification. |
| Hepatitis C | |
| HCV antibody (anti-HCV) | Indicates current hepatitis C [in someone with a positive hepatitis C virus (HCV) detection test] or past HCV infection (in someone with a negative HCV detection test). On average, becomes detectable by current HCV immunoassay tests approximately 8–11 weeks post HCV exposure. |
| Hepatitis C virus ribonucleic acid (HCV RNA) | Indicates current HCV infection. Also indicates chronic hepatitis C in those who have detectable HCV RNA for >6 months. On average, HCV RNA becomes detectable approximately 1–2 weeks post HCV exposure. HCV RNA represents the most sensitive and specific indicator of current infection, and if quantified, correlates with levels of HCV in serum or plasma, measured in IUI/mL. |
| HCV core antigen | Indicates current HCV infection. Also indicates chronic HCV infection in those who are positive for HCV core antigen for ≥6 months. Can be used as an alternative to HCV RNA as an indicator of active infection. No HCV core antigen tests have approved by the US Food and Drug Administration (FDA). When these tests become available, they will serve as acceptable laboratory criteria for active infection, equivalent to HCV RNA testing. |
| HCV genotype |
Categorizes the specific HCV genetic strain, which can affect the natural history of chronic infection. When a genotype is determined, it indicates current HCV infection.
|
Appendix C. Classification Scenarios for Cases of Hepatitis A, Hepatitis B, and Hepatitis C
Cases of hepatitis A; acute, chronic, and perinatal hepatitis B; and acute, chronic, and perinatal hepatitis C should be classified in accordance with their respective CDC/CSTE surveillance case definition. The scenarios provided in the following tables can serve as guidance for classification of these cases. Technical assistance is available for more complex scenarios by contacting the assigned regional CDC DVH technical assistance team.
Classification Scenarios for Cases of Acute Hepatitis B
Classification Scenarios for Cases of Chronic Hepatitis B
Classification Scenarios for Cases of Perinatal Hepatitis B
Classification Scenarios for Cases of Acute Hepatitis C
Classification Scenarios for Cases of Chronic Hepatitis C
Classification Scenarios for Cases of Perinatal Hepatitis C
Classification Scenarios for Cases of Hepatitis A
Scenario 1: A primary care provider reported a case of hepatitis A. The patient had a positive anti-HAV IgM test result, and the provider reported abdominal pain, dark urine, and nausea. Liver function tests show a total bilirubin level of 6.2 mg/dL, and there is not a more likely diagnosis than hepatitis A. You were not able to find this patient in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the laboratory or epidemiologic linkage criterion? | ||
| Lab criterion 1: anti-HAV IgM | Positive |
Needs to meet at least 1: ☒ Positive anti-HAV IgM |
| Lab criterion 2: HAV RNA | Unknown | |
| Epidemiologic linkage: Contact with lab-confirmed hepatitis A case during exposure period | Unknown | |
| Does this meet all 3 clinical criteria? | ||
| Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Present |
Needs to meet all 3 unless lab criterion 2 is met: ☒ Jaundice or elevated total bilirubin or elevated ALT |
| Clinical criterion 2: Distinct episode of symptoms consistent with acute viral hepatitis | Present | |
| Clinical criterion 3: Alternate, more likely diagnosis | Absent | |
| Is this a new event? | ||
| Is the patient newly reported (i.e., not a relapse case)? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient meets the classification criteria for confirmed hepatitis A | ||
Scenario 2: The HD received a positive HAV RNA laboratory result from a local plasma donation center. The patient does not have a more likely diagnosis than hepatitis A, and the donor was not located in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the laboratory or epidemiologic linkage criterion? | ||
| Lab criterion 1: anti-HAV IgM | Unknown |
Needs to meet at least 1: ☐ Positive anti-HAV IgM |
| Lab criterion 2: HAV RNA | Positive | |
| Epidemiologic linkage: Contact with lab-confirmed hepatitis A case during exposure period | Unknown | |
| Does this meet all 3 clinical criteria? | ||
| Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Not needed |
Needs to meet all 3 unless lab criteria 2 is met: ☐ Jaundice or elevated total bilirubin or elevated ALT |
| Clinical criterion 2: Distinct episode of symptoms consistent with acute viral hepatitis | Not needed | |
| Clinical criterion 3: Alternate, more likely diagnosis | Absent | |
| Is this a new event? | ||
| Is the patient newly reported (i.e., not a relapse case)? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient meets the classification criteria for confirmed hepatitis A. | ||
Scenario 3: The HD received an electronic laboratory report from a hospital for a patient who is positive for anti-HAV IgM. After speaking with the infection preventionist, the surveillance staff member learned that the patient has symptoms of vomiting, abdominal pain, and weakness. Liver function tests show an ALT level of 1,347 IU/L, and there is a diagnosis of acetaminophen toxicity.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the laboratory or epidemiologic linkage criterion? | ||
| Lab criterion 1: anti-HAV IgM | Present |
Needs to meet at least 1: ☒ Positive anti-HAV IgM |
| Lab criterion 2: HAV RNA | Unknown | |
| Epidemiologic linkage: Contact with lab-confirmed hepatitis A case during exposure period | Unknown | |
| Does this meet all 3 clinical criteria? | ||
| Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Present |
Need to meet all 3 unless lab criteria 2 is met: ☒ Jaundice or elevated total bilirubin or elevated ALT |
| Clinical criterion 2: Distinct episode of symptoms consistent with acute viral hepatitis | Present | |
| Clinical criterion 3: Alternate, more likely diagnosis | Present | |
| Is this a new event? | ||
| Is the patient newly reported (i.e., not a relapse case)? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient does not meet the classification criteria for confirmed hepatitis A. | ||
Classification Scenarios for Cases of Acute Hepatitis B
Scenario 1: A HD received positive HBsAg and anti-HBc IgM test results on a patient 49 years of age. The peak ALT level associated with the positive HBsAg and anti-HBc IgM test results was 1,087 IU/L. The patient was not found in the surveillance system as an acute or chronic hepatitis B case.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 49 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet confirmatory laboratory criteria for acute hepatitis B? | Tier 1 lab criterion 1: Detection of HBsAg, HBeAg, or HBV DNA AND detection of anti-HBc IgM | Positive HBsAg and positive anti-HBc IgM |
Needs to meet 1 lab criterion (any from tier 1 or 2): ☒ Detection of HBsAg, HBeAg, or HBV DNA AND detection of anti-HBc IgM ☐ Documented HBsAg test conversion |
| Tier 1 lab criterion 2: HBsAg test conversion from negative to positive within 12 months* | Not documented | ||
| Tier 2 lab criterion: Detection of HBsAg or HBV DNA AND anti-HBc IgM not done or not available | Positive HBsAg and positive anti-HBc IgM | ☐ Detection of HBsAg or HBV DNA AND anti-HBc IgM not done or not available | |
| Does this meet clinical criteria? | Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Present |
If the patient meets the tier 2 lab criterion, they must also meet the clinical criteria in the absence of a more likely diagnosis: ☒ Jaundice or elevated total bilirubin or elevated ALT ☒ Absence of a more likely diagnosis |
| Clinical criterion 2: Alternate, more likely diagnosis | Absent | ||
| Is this a new event? | New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
*Negative HBsAg laboratory test within 6 months prior to a positive test for either HBsAg, HBeAg, or HBV DNA (including qualitative, quantitative, or genotype).
Classification: This patient meets the classification criteria for confirmed acute hepatitis B.
Scenario 2: The HD received a positive HBsAg laboratory result from a regular donor 52 years of age at a local plasma donation center in June. The patient had a negative HBsAg laboratory result in October of the prior year.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 52 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet confirmatory laboratory criteria for acute hepatitis B? | Tier 1 lab criterion 1: Detection of HBsAg, HBeAg, or HBV DNA AND detection of anti-HBc IgM | Positive HBsAg and positive anti-HBc IgM |
Needs to meet 1 lab criterion (any from tier 1 or 2): ☐ Detection of HBsAg, HBeAg, or HBV DNA AND detection of anti-HBc IgM ☒ Documented HBsAg test conversion |
| Tier 1 lab criterion 2: HBsAg test conversion from negative to positive within 12 months* | Documented | ||
| Tier 2 lab criterion: Detection of HBsAg or HBV DNA AND anti-HBc IgM not done or not available | Positive HBsAg and unknown anti-HBc IgM | ☒ Detection of HBsAg or HBV DNA AND anti-HBc IgM not done or not available | |
| Does this meet clinical criteria? | Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | N/A |
If the patient meets the tier 2 lab criterion, they must also meet the clinical criteria in the absence of a more likely diagnosis: ☐ Jaundice or elevated total bilirubin or elevated ALT ☐ Absence of a more likely diagnosis |
| Clinical criterion 2: Alternate, more likely diagnosis | N/A | ||
| Is this a new event? | New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
*Negative HBsAg laboratory test within 12 months prior to a positive test for either HBsAg, HBeAg, or HBV DNA (including qualitative, quantitative, or genotype).
Classification: This patient meets the classification criteria for confirmed acute hepatitis B.
Scenario 3: The HD received a positive HBsAg laboratory result on a person 37 years of age. The test result for anti-HBc IgM was either not done or not reported to the HD. Liver function tests show an ALT level of 226 IU/L. There is not a more likely diagnosis than acute hepatitis B to explain the elevated ALT level. The patient was not an acute or chronic hepatitis B case in the surveillance system.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 37 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet confirmatory laboratory criteria for acute hepatitis B? | Tier 1 lab criterion 1: Detection of HBsAg, HBeAg, or HBV DNA AND detection of anti-HBc IgM | Positive HBsAg and unknown anti-HBc IgM |
Needs to meet 1 lab criterion (any from tier 1 or 2): ☐ Detection of HBsAg, HBeAg, or HBV DNA AND detection of anti-HBc IgM ☐ Documented HBsAg test conversion |
| Tier 1 lab criterion 2: HBsAg test conversion from negative to positive within 12 months* | Not Documented | ||
| Tier 2 lab criterion: Detection of HBsAg or HBV DNA AND anti-HBc IgM not done or not available | Positive HBsAg and unknown anti-HBc IgM | ☒ Detection of HBsAg or HBV DNA AND anti-HBc IgM not done or not available | |
| Does this meet clinical criteria? | Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | ALT was 226 IU/L |
If the patient meets the tier 2 lab criterion, they must also meet the clinical criteria in the absence of a more likely diagnosis: ☒ Jaundice or elevated total bilirubin or elevated ALT ☒ Absence of a more likely diagnosis |
| Clinical criterion 2: Alternate, more likely diagnosis | Absent | ||
| Is this a new event? | New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
*Negative HBsAg laboratory test within 12 months prior to a positive test for either HBsAg, HBeAg, or HBV DNA (including qualitative, quantitative, or genotype).
Classification: This patient meets the classification criteria for confirmed acute hepatitis B.
Scenario 4: The HD received a positive anti-HBc IgM laboratory result on a person 38 years of age. The surveillance staff contacted the ordering provider and determined that the patient tested negative for HBsAg and positive for total anti-HBc. The patient does have a provider report of jaundice. There is not a more likely diagnosis than acute hepatitis B to explain the presence of jaundice. The patient was not an acute or chronic hepatitis B case in the surveillance system.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 38 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet presumptive laboratory criteria for acute hepatitis B? | Lab criterion 1: Detection of anti-HBc IgM AND negative or not done for HBsAg, HBeAg, or HBV DNA | Positive anti-HBc IgM and negative HBsAg |
Needs to meet lab criterion: ☒ Detection of anti-HBc IgM AND negative or not done for HBsAg, HBeAg, or HBV DNA
Must also meet clinical criteria in the absence of a more likely diagnosis: ☒ Jaundice or elevated total bilirubin or elevated ALT ☒ Absence of a more likely diagnosis |
| Does this meet clinical criteria? | Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Provider report of jaundice | |
| Clinical criterion 2: Alternate, more likely diagnosis | Absent | ||
| Is this a new event? | New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
*Negative HBsAg laboratory test within 6 months prior to a positive test for either HBsAg, HBeAg, or HBV DNA (including qualitative, quantitative, or genotype).
Classification: This patient meets the classification criteria for probable acute hepatitis B.
Classification Scenarios for Cases of Chronic Hepatitis B
Scenario 1: The HD received a single positive HBsAg laboratory result on a person who was 28 years of age. The test result for anti-HBc IgM was negative. No other information was available. The patient was not an acute or chronic hepatitis B case in the surveillance system.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 28 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet the confirmatory laboratory criteria for chronic hepatitis B? | Lab criterion 1: Detection of HBsAg in 2 clinical specimens taken >6 months apart | No (single positive HBsAg) |
Confirmed if meets 1 diagnostic lab criterion: ☐ Detection of HBsAg in 2 clinical specimens taken >6 months apart ☐ Detection of HBeAg in 2 clinical specimens taken >6 months apart ☐ Detection of [HBsAg or HBeAg] AND detection of total anti-HBc ☐ Detection of HBsAg AND HBeAg ☐ Detection of HBV DNA |
| Lab criterion 2: Detection of HBeAg in 2 clinical specimens taken >6 months apart | No (unknown HBeAg result) | ||
| Lab criterion 3: Detection of [HBsAg or HBeAg] AND detection of total anti-HBc | No (unknown total anti-HBc result) | ||
| Lab criterion 4: Detection of HBsAg AND HBeAg | No (single positive HBsAg) | ||
| Lab criterion 5: Detection of HBV DNA | No (unknown HBV DNA result) | ||
| Does this meet the presumptive laboratory criterion for chronic hepatitis B? | Lab criterion 1: Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available | Positive HBsAg and negative anti-HBc IgM |
Probable if meets presumptive lab criterion: ☒ Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available (and does not meet the case definition for acute hepatitis B) |
| Is this a new event? | New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion: ☒ Newly reported ☐ Previously reported as an acute event AND the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result ☐ Previously reported as a probable chronic hepatitis B event |
| New event criterion 2: Does the patient have an acute hepatitis B event in the surveillance system and the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result? | No | ||
| New event criterion 3: Does the patient exist as a probable chronic hepatitis B event in the surveillance system? | No | ||
| Classification: This patient meets the classification criteria for probable chronic hepatitis B. | |||
Scenario 2: The 28-year-old patient who was classified as a probable chronic hepatitis B case in Scenario 1 was linked to medical care and was tested for HBsAg 7 months later to confirm the chronic HBV infection. After laboratory testing, a positive HBsAg result was reported to the HD. The patient was an existing probable chronic hepatitis B case in the HD’s surveillance system.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 28 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet the confirmatory laboratory criteria for chronic hepatitis B? | Lab criterion 1: Detection of HBsAg in 2 clinical specimens taken >6 months apart | Yes, 2 positive HBsAg results in 2 clinical specimens taken 7 months apart |
Confirmed if meets 1 diagnostic lab criterion: ☒ Detection of HBsAg in 2 clinical specimens taken >6 months apart ☐ Detection of HBeAg in 2 clinical specimens taken >6 months apart ☐ Detection of [HBsAg or HBeAg] AND detection of total anti-HBc ☐ Detection of HBsAg AND HBeAg ☐ Detection of HBV DNA |
| Lab criterion 2: Detection of HBeAg in 2 clinical specimens taken >6 months apart | No (unknown HBeAg result) | ||
| Lab criterion 3: Detection of [HBsAg or HBeAg] AND detection of total anti-HBc | No (unknown total anti-HBc result) | ||
| Lab criterion 4: Detection of HBsAg AND HBeAg | No (single positive HBsAg) | ||
| Lab criterion 5: Detection of HBV DNA | No (unknown HBV DNA result) | ||
| Does this meet the presumptive laboratory criterion for chronic hepatitis B? | Lab criterion 1: Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available | Positive HBsAg and unknown anti-HBc IgM result |
Probable if meets presumptive lab criterion: ☐ Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available (and does not meet the case definition for acute hepatitis B) |
| Is this a new event? | New event criterion 1: Is the patient newly reported? | No |
Needs to meet 1 new event criterion: ☐ Newly reported ☐ Previously reported as an acute event AND the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result ☒ Previously reported as a probable chronic hepatitis B event |
| New event criterion 2: Does the patient have an acute hepatitis B event and the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result? | No | ||
| New event criterion 3: Does the patient exist as a probable chronic hepatitis B event in the surveillance system? | Yes | ||
| Classification: This patient meets the classification criteria for confirmed chronic hepatitis B. Reclassify the case and update the case notification from probable chronic hepatitis B to confirmed chronic hepatitis B. | |||
Scenario 3: The HD received a positive HBsAg laboratory result on a person 42 years of age. The total anti-HBc result was also positive. The case report form filled out by the provider did not indicate that the patient had any clinical signs of acute viral hepatitis. The patient was not an acute or chronic hepatitis B case in the surveillance system.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 42 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet the confirmatory laboratory criteria for chronic hepatitis B? | Lab criterion 1: Detection of HBsAg in 2 clinical specimens taken >6 months apart | No (single positive HBsAg) |
Confirmed if meets 1 diagnostic lab criterion: ☐ Detection of HBsAg in 2 clinical specimens taken >6 months apart ☐ Detection of HBeAg in 2 clinical specimens taken >6 months apart ☒ Detection of [HBsAg or HBeAg] AND detection of total anti-HBc ☐ Detection of HBsAg AND HBeAg ☐ Detection of HBV DNA |
| Lab criterion 2: Detection of HBeAg in 2 clinical specimens taken >6 months apart | No (unknown HBeAg result) | ||
| Lab criterion 3: Detection of [HBsAg or HBeAg] AND detection of total anti-HBc | Yes (positive HBsAg and positive total anti-HBc) | ||
| Lab criterion 4: Detection of HBsAg AND HBeAg | No (single positive HBsAg) | ||
| Lab criterion 5: Detection of HBV DNA | No (unknown HBV DNA result) | ||
| Does this meet the presumptive laboratory criterion for chronic hepatitis B? | Lab criterion 1: Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available | Positive HBsAg and unknown anti-HBc IgM result |
Probable if meets presumptive lab criterion: ☐ Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available (and does not meet the case definition for acute hepatitis B) |
| Is this a new event? | New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion: ☒ Newly reported ☐ Previously reported as an acute event AND the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result ☐ Previously reported as a probable chronic hepatitis B event |
| New event criterion 2: Does the patient have an acute hepatitis B event in the surveillance system and the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result? | No | ||
| New event criterion 3: Does the patient exist as a probable chronic hepatitis B event in the surveillance system? | No | ||
| Classification: This patient meets the classification criteria for confirmed chronic hepatitis B. | |||
Scenario 4: The HD received a detectable HBV DNA laboratory result on a 42-year-old patient that is already in the HD’s surveillance system as a confirmed acute hepatitis B case (positive for anti-IgM and HBsAg). It has been 12 months since the initial positive test results.
| Criteria Category | Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|---|
| Does this meet age criterion? | Age criterion: >24 months of age (or mode of exposure is not perinatal if <24 months of age) | 42 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet the confirmatory laboratory criteria for chronic hepatitis B? | Lab criterion 1: Detection of HBsAg in 2 clinical specimens taken >6 months apart | No |
Confirmed if meets 1 diagnostic lab criterion: ☐ Detection of HBsAg in 2 clinical specimens taken >6 months apart ☐ Detection of HBeAg in 2 clinical specimens taken >6 months apart ☐ Detection of [HBsAg or HBeAg] AND detection of total anti-HBc ☐ Detection of HBsAg AND HBeAg ☒ Detection of HBV DNA |
| Lab criterion 2: Detection of HBeAg in 2 clinical specimens taken >6 months apart | No | ||
| Lab criterion 3: Detection of [HBsAg or HBeAg] AND detection of total anti-HBc | No | ||
| Lab criterion 4: Detection of HBsAg AND HBeAg | No | ||
| Lab criterion 5: Detection of HBV DNA | Yes (detectable HBV DNA) | ||
| Does this meet the presumptive laboratory criterion for chronic hepatitis B? | Lab criterion 1: Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available | No |
Probable if meets presumptive lab criterion: ☐ Detection of [HBsAg or HBeAg] AND anti-HBc IgM test is negative, not done, or not available (and does not meet the case definition for acute hepatitis B) |
| Is this a new event? | New event criterion 1: Is the patient newly reported? | No |
Needs to meet 1 new event criterion: ☐ Newly reported ☒ Previously reported as an acute event AND the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result ☐ Previously reported as a probable chronic hepatitis B event |
| New event criterion 2: Does the patient have an acute hepatitis B event and the chronic event occurred >6 months after acute case onset, or if asymptomatic, after the initial positive test result? | Yes | ||
| New event criterion 3: Does the patient exist as a probable chronic hepatitis B event in the surveillance system? | No | ||
| Classification: This patient meets the classification criteria for confirmed chronic hepatitis B. A confirmed acute hepatitis B case may be additionally enumerated as a new confirmed chronic hepatitis B case if a positive HBV viral detection test is reported 6 months or longer after acute case onset, or if asymptomatic, after the initial positive test result. | |||
Classification Scenarios for Cases of Perinatal Hepatitis B
Scenario 1: A provider contacted the HD to report a positive HBsAg test result in an infant 12 months of age. The infant’s gestational parent was positive for HBsAg at the time of delivery, and the birth occurred at a local hospital. The infant’s anti-HBs result, also performed at 12 months of age as part of PVST, was negative. The infant could not be matched with an existing case of hepatitis B in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet demographic criteria? | ||
| Demographic criterion 1: 1–24 months of age | 12 months of age |
Needs to meet both demographic criteria: ☒ 1–24 months of age |
| Demographic criterion 2: Birth occurred in the United States | Documented | |
| Does this meet epidemiologic linkage criterion? | ||
| Epidemiologic linkage criterion: Birth to an HBV-infected gestational parent | Documented |
Confirmed if case meets epidemiologic linkage criterion; probable if case does not meet epidemiologic linkage criterion: ☒ Epidemiologic linkage criterion |
| Does this meet laboratory criteria? | ||
| Lab criterion 1: HBsAg during 1–24 months of age | Positive |
Needs to meet at least 1 lab criterion: ☒ Positive HBsAg during 1–24 months of age |
| Lab criterion 2: HBeAg during 9–24 months of age | Unknown | |
| Lab criterion 3: HBV DNA during 9–24 months of age | Unknown | |
| Is this a new event? | ||
| New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient meets the classification criteria for confirmed perinatal hepatitis B. | ||
Scenario 2: A provider contacted the HD to report a positive HBsAg test result in an infant 9 months of age. The test was performed 2 months after receipt of the last dose of hepatitis B vaccine, and the birth occurred at a local hospital. Information could not be obtained on the gestational parent’s HBsAg or HBV DNA status at the time of delivery. The infant could not be matched with an existing case of hepatitis B in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet both demographic criteria? | ||
| Demographic criterion 1: 1–24 months of age | 9 months of age |
Needs to meet both demographic criteria: ☒ 1–24 months of age |
| Demographic criterion 2: Birth occurred in the United States | Documented | |
| Does this meet the epidemiologic linkage criterion? | ||
| Epidemiologic linkage criterion: Birth to an HBV-infected gestational parent | Unknown |
Confirmed if case meets epidemiologic linkage criterion; probable if case does not meet epidemiologic linkage criterion: ☐ Epidemiologic linkage criterion |
| Does this meet at least 1 laboratory criterion? | ||
| Lab criterion 1: HBsAg during 1–24 months of age | Positive |
Needs to meet at least 1 lab criterion: ☒ Positive HBsAg during 1–24 months of age |
| Lab criterion 2: HBeAg during 9–24 months of age | Unknown | |
| Lab criterion 3: HBV DNA during 9–24 months of age | Unknown | |
| Is this a new event? | ||
| New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient meets the classification criteria for probable perinatal hepatitis B. This case may be reclassified as confirmed perinatal hepatitis B if the gestational parent’s HBsAg or HBV DNA status is verified to be positive. | ||
Scenario 3: The HD received a positive HBV DNA laboratory result on a child 18 months of age who recently immigrated to the United States. Through follow-up investigation, it was determined that the gestational parent has chronic hepatitis B. The infant could not be matched with an existing case of hepatitis B in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet both demographic criteria? | ||
| Demographic criterion 1: 1–24 months of age | 18 months of age |
Needs to meet both demographic criteria: ☒ 1–24 months of age |
| Demographic criterion 2: Birth occurred in the United States | No | |
| Does this meet the epidemiologic linkage criterion? | ||
| Epidemiologic linkage criterion: Birth to an HBV-infected gestational parent | Documented |
Confirmed if case meets epidemiologic linkage criterion; probable if case does not meet epidemiologic linkage criterion: ☒ Epidemiologic linkage criterion |
| Does this meet at least 1 laboratory criterion? | ||
| Lab criterion 1: HBsAg during 1–24 months of age | Unknown |
Needs to meet at least 1 lab criterion: ☐ Positive HBsAg during 1–24 months of age |
| Lab criterion 2: HBeAg during 9–24 months of age | Unknown | |
| Lab criterion 3: HBV DNA during 9–24 months of age | Positive | |
| Is this a new event? | ||
| New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient does not meet the classification criteria for either confirmed or probable perinatal hepatitis B. Children 1–24 months of age whose birth location was unknown or occurred outside of the United States should be classified under the acute or chronic hepatitis B case definition, even if other criteria categories were met. | ||
Classification Scenarios for Cases of Acute Hepatitis C
Scenario 1: A primary care provider reported a positive HCV RNA test result in a person 24 years of age. Liver function tests show a peak ALT level of 236 IU/L, but jaundice is not present. There is not a more likely diagnosis than acute hepatitis C. The patient could not be matched with an existing acute or chronic case of hepatitis C in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 24 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory lab criterion: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen) | Positive |
Confirmed if meets confirmatory or test conversion lab criterion; probable if meets only presumptive lab criterion: ☒ Positive HCV detection test |
| Presumptive lab criterion: HCV antibody test | Unknown | |
| Test conversion lab criterion: HCV antibody or HCV detection* test conversion from negative to positive within 12 months | Not documented | |
| Does this meet clinical criteria? | ||
| Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Present |
Needs to meet both criteria unless anti-HCV or HCV detection test conversion criterion is met: ☒ Jaundice or peak elevated total bilirubin or peak elevated ALT |
| Clinical criterion 2: Alternate, more likely diagnosis | Absent | |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion†: ☒ Newly reported |
| New event criterion 2: Does the patient have an existing acute or chronic hepatitis C event with evidence of reinfection? | No | |
| Classification: This patient meets the classification criteria for confirmed acute hepatitis C. | ||
†Some jurisdictions are creating a local condition specific for reinfection as opposed to creating a new acute condition to maintain a deduplicated registry.
Scenario 2: The HD received a positive anti-HCV laboratory result from an SSP in December on a person 39 years of age. The HD collects negative hepatitis C (anti-HCV and HCV detection) laboratory results. The patient had a previous negative anti-HCV laboratory result in February.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 39 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory lab criterion: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen) | Unknown |
Confirmed if meets confirmatory or test conversion lab criterion; probable if meets only presumptive lab criterion: ☐ Positive HCV detection test |
| Presumptive lab criterion: HCV antibody test | Positive | |
| Test conversion lab criterion: HCV antibody or HCV detection* test conversion from negative to positive within 12 months | Documented | |
| Does this meet clinical criteria? | ||
| Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Unknown |
Needs to meet both criteria unless anti-HCV unless HCV detection test conversion criterion is met: ☐ Jaundice or peak elevated total bilirubin or peak elevated ALT |
| Clinical criterion 2: Alternate, more likely diagnosis | Not required | |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion†: ☒ Newly reported |
| New event criterion 2: Does the patient have an existing acute or chronic hepatitis C event with evidence of reinfection? | No | |
| Classification: This patient meets the classification criteria for confirmed acute hepatitis C. | ||
†Some jurisdictions are creating a local condition specific for reinfection as opposed to creating a new acute condition to maintain a deduplicated registry.
Scenario 3: In September, the HD received a positive HCV RNA laboratory result on a person 42 years of age. Three months later, the HD received another positive HCV RNA laboratory result on the same person. The HD collects negative hepatitis C (anti-HCV and HCV detection) laboratory results. The patient was matched with an existing chronic hepatitis C case from a previous year, and there are two subsequent negative HCV RNA laboratory results 3 months apart, indicating a cleared HCV infection.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 42 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory lab criterion: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen) | Positive |
Confirmed if meets confirmatory or test conversion lab criterion; probable if meets only presumptive lab criterion: ☒ Positive HCV detection test |
| Presumptive lab criterion: HCV antibody test | Positive | |
| Test conversion lab criterion: HCV antibody or HCV detection* test conversion from negative to positive within 12 months | Documented | |
| Does this meet clinical criteria? | ||
| Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Unknown |
Needs to meet both criteria unless anti-HCV or HCV detection test conversion criterion is met: ☐ Jaundice or peak elevated total bilirubin or peak elevated ALT |
| Clinical criterion 2: Alternate, more likely diagnosis | Unknown | |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | No |
Needs to meet 1 new event criterion†: ☐ Newly reported |
| New event criterion 2: Does the patient have an existing acute or chronic hepatitis C event with evidence of reinfection? | Yes | |
| Classification: This patient meets the classification criteria for confirmed acute hepatitis C†. | ||
†Some jurisdictions are creating a local condition specific for reinfection as opposed to creating a new acute condition to maintain a deduplicated registry.
Scenario 4: The HD received a positive anti-HCV laboratory result on a person 20 years of age. The person’s HCV RNA status is unknown. Through provider follow-up, it was determined that the patient presented with nausea, fatigue, and jaundice; the peak ALT level was 642 IU/L. There is not a more likely diagnosis than acute hepatitis C. This patient could not be matched with an existing acute or chronic case of hepatitis C in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 20 years of age |
Needs to meet age criterion ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory lab criterion: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen) | Unknown |
Confirmed if meets confirmatory or test conversion lab criterion; probable if meets only presumptive lab criterion: ☐ Positive HCV detection test |
| Presumptive lab criterion: HCV antibody test | Positive | |
| Test conversion lab criterion: HCV antibody or HCV detection* test conversion from negative to positive within 12 months | Not documented | |
| Does this meet clinical criteria? | ||
| Clinical criterion 1: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L | Present |
Needs to meet both criteria unless anti-HCV or HCV detection test conversion criterion is met: ☒ Jaundice or peak elevated total bilirubin or peak elevated ALT |
| Clinical criterion 2: Alternate, more likely diagnosis | Absent | |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion†: ☒ Newly reported |
| New event criterion 2: Does the patient have an existing acute or chronic hepatitis C event with evidence of reinfection? | No | |
| Classification: This patient meets the classification criteria for probable acute hepatitis C. | ||
†Some jurisdictions are creating a local condition specific for reinfection as opposed to creating a new acute condition to maintain a deduplicated registry.
Classification Scenarios for Cases of Chronic Hepatitis C
Scenario 1: The HD received a laboratory result on a person 62 years of age who has a positive anti-HCV result and a positive HCV RNA result. Total bilirubin level was 0.2 mg/dL, and ALT was 22 IU/L. The patient could not be matched with an existing acute or chronic case of hepatitis C in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 62 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory laboratory evidence: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen | Positive |
Confirmed if meets confirmed lab case classification; probable if meets probable lab case classification: ☒ Positive HCV detection test and has no documentation of HCV antibody or HCV detection test conversion within 12 months |
| Presumptive laboratory evidence: HCV antibody test | Positive | |
| Does this meet clinical criteria? | ||
| Clinical criteria: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L and the absence of a more likely diagnosis | No |
Does not meet or has no report of clinical criteria: ☐ Jaundice or peak elevated total bilirubin or peak elevated ALT and the absence of a more likely diagnosis |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion: ☒ Newly reported |
| New event criterion 2: Does the patient have an acute hepatitis C event in the surveillance system in a previous MMWR year and >1 year after acute onset? | No | |
| Classification: This patient meets the classification criteria for confirmed chronic hepatitis C. | ||
*HCV detection tests include a nucleic acid test (NAT) for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Scenario 2: The HD received an HCV genotype laboratory result on a person 38 years of age. The patient was matched to an existing acute hepatitis C case in your jurisdiction’s surveillance system from 18 months prior (i.e., positive HCV antibody with positive reflexed HCV RNA).
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 38 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory laboratory evidence: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen | Positive |
Confirmed if meets confirmed lab case classification; probable if meets probable lab case classification: ☒ Positive HCV detection test and has no documentation of HCV antibody or HCV detection test conversion within 12 months |
| Presumptive laboratory evidence: HCV antibody test | Positive | |
| Does this meet clinical criteria? | ||
| Clinical criteria: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L and the absence of a more likely diagnosis | No |
Does not meet or has no report of clinical criteria: ☐ Jaundice or peak elevated total bilirubin or peak elevated ALT and the absence of a more likely diagnosis |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | No |
Needs to meet 1 new event criterion: ☐ Newly reported |
| New event criterion 2: Does the patient have an acute hepatitis C event in the surveillance system in a previous MMWR year and >1 year after acute onset? | Yes | |
| Classification: This patient meets the classification criteria for confirmed chronic hepatitis C. | ||
*HCV detection tests include a nucleic acid test (NAT) for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Scenario 3: The HD received a positive anti-HCV laboratory result on a person 38 years of age from an SSP. Upon contacting the SSP, it was learned that the patient did not show up to the referring provider for HCV RNA follow-up testing. Liver function tests were not available. This patient could not be matched with an existing acute or chronic case of hepatitis C in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 38 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory laboratory evidence: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen | Unknown |
Confirmed if meets confirmed lab case classification; probable if meets probable lab case classification: ☐ Positive HCV detection test and has no documentation of HCV antibody or HCV detection test conversion within 12 months |
| Presumptive laboratory evidence: HCV antibody test | Positive | |
| Does this meet clinical criteria? | ||
| Clinical criteria: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L and the absence of a more likely diagnosis | Unknown |
Does not meet or has no report of clinical criteria: ☐ Jaundice or peak elevated total bilirubin or peak elevated ALT and the absence of a more likely diagnosis |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion: ☒ Newly reported |
| New event criterion 2: Does the patient have an acute hepatitis C event in the surveillance system in a previous MMWR year and >1 year after acute onset? | No | |
| Classification: This patient meets the classification criteria for probable chronic hepatitis C. If resources permit, staff who are coordinating linkage-to-cure activities should follow-up with the patient to offer referral to care, follow-up testing, and other services, as needed. | ||
*HCV detection tests include a nucleic acid test (NAT) for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Scenario 4: The HD received a positive anti-HCV laboratory result and a positive HCV RNA laboratory result on a pregnant person 25 years of age. Liver function tests were not elevated. The patient could not be matched with an existing acute or chronic case of hepatitis C in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: >36 months of age (or mode of exposure is not perinatal if 2–36 months of age) | 25 years of age |
Needs to meet age criterion: ☒ Age criterion |
| Does this meet laboratory criteria? | ||
| Confirmatory laboratory evidence: HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen | Positive |
Confirmed if meets confirmed lab case classification; probable if meets probable lab case classification: ☒ Positive HCV detection test and has no documentation of HCV antibody or HCV detection test conversion within 12 months |
| Presumptive laboratory evidence: HCV antibody test | Positive | |
| Does this meet clinical criteria? | ||
| Clinical criteria: Jaundice or peak elevated total bilirubin ≥3.0 mg/dL or peak elevated ALT >200 IU/L and the absence of a more likely diagnosis | No |
Does not meet or has no report of clinical criteria: ☐ Jaundice or peak elevated total bilirubin or peak elevated ALT and the absence of a more likely diagnosis |
| Is this a new event? | ||
| New event criterion 1: Is the patient newly reported? | Yes |
Needs to meet 1 new event criterion: ☒ Newly reported |
| New event criterion 2: Does the patient have an acute hepatitis C event in the surveillance system in a previous MMWR year and >1 year after acute onset? | No | |
| Classification: This patient meets the classification criteria for confirmed chronic hepatitis C. Depending on local protocols, a jurisdiction might additionally open a perinatal hepatitis C case investigation record with a non-notifiable classification such as ‘suspected’ as a reminder in the system that the infant’s HCV infection test results will need be obtained. The infant’s HCV detection test results performed during 2–36 months of age will determine whether the infant will be classified as confirmed perinatal hepatitis C. | ||
*HCV detection tests include a nucleic acid test (NAT) for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Classification Scenarios for Cases of Perinatal Hepatitis C
Scenario 1: A provider contacted the HD to report a positive HCV RNA test result in a child 6 months of age. Through birth certificate matching, the gestational parent was reported as a chronic hepatitis C case in the surveillance system. No evidence of another likely mode of transmission exists other than perinatal. The child is not an existing hepatitis C case in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet age criterion? | ||
| 2–36 months of age | 6 months of age |
Needs to meet age criterion: ☒ 2–36 months of age |
| Does this meet the epidemiologic linkage criterion? | ||
| Not known to have been exposed to hepatitis C via a mechanism other than perinatal | Documented |
Needs to meet epidemiologic linkage criterion: ☒ Epidemiologic linkage criteria |
| Does this meet the laboratory criterion? | ||
| HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen) during 2–36 months of age | Positive |
Needs to meet laboratory criterion: ☒ Positive HCV detection test during 2–36 months of age |
| Is this a new event? | ||
| New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient meets the classification criteria for confirmed perinatal hepatitis C. | ||
*HCV detection tests include a nucleic acid test (NAT) for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Scenario 2: The HD received a positive anti-HCV laboratory result on a child 24 months of age. Through follow-up with the ordering provider, the gestational parent’s information was obtained. The gestational parent is a confirmed chronic hepatitis C case in the surveillance system. The HCV detection status of the child is unknown, and the gestational parent was lost to follow-up. The child could not be matched to an existing hepatitis C case in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: 2–36 months of age | 24 months of age |
Needs to meet age criterion: ☒ 2–36 months of age |
| Does this meet the epidemiologic linkage criterion? | ||
| Epidemiologic linkage criterion: Not known to have been exposed to hepatitis C via a mechanism other than perinatal | Documented |
Needs to meet epidemiologic linkage criterion: ☒ Epidemiologic linkage criteria |
| Does this meet the laboratory criterion? | ||
| HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen) during 2–36 months of age | Unknown |
Needs to meet laboratory criterion: ☐ Positive HCV detection test during 2–36 months of age |
| Is this a new event? | ||
| New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient does not meet the classification criteria for confirmed perinatal hepatitis C. Though there is laboratory evidence of perinatal exposure (i.e., positive anti-HCV result 18–36 months of age), HCV detection is needed for confirmation. HDs might consider classifying the patient as ‘suspected’ as a way to hold the patient in the surveillance system for receipt of the HCV detection test result. | ||
*HCV detection tests include a nucleic acid test (NAT) for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Scenario 3: The HD received a positive anti-HCV laboratory result on a child 24 months of age. Through follow-up with the ordering provider, the gestational parent’s information was obtained. The gestational parent is a confirmed chronic hepatitis C case in the surveillance system. Upon further investigation, the HCV RNA status of the child is negative. The child could not be matched to an existing hepatitis C case in the surveillance system.
| Case Classification Criteria | Scenario | Rationale for Classification |
|---|---|---|
| Does this meet the age criterion? | ||
| Age criterion: 2–36 months of age | 24 months of age |
Needs to meet age criterion: ☒ 2–36 months of age |
| Does this meet the epidemiologic linkage criterion? | ||
| Epidemiologic linkage criterion: Not known to have been exposed to hepatitis C via a mechanism other than perinatal | Documented |
Needs to meet epidemiologic linkage criterion: ☒ Epidemiologic linkage criteria |
| Does this meet the laboratory criterion? | ||
| HCV detection* test (i.e., HCV RNA, HCV genotype, or HCV antigen) during 2–36 months of age | Negative |
Needs to meet laboratory criterion: ☐ Positive HCV detection test during 2–36 months of age |
| Is this a new event? | ||
| New event criterion: Is the patient newly reported? | Yes |
Needs to be newly reported event: ☒ Newly reported |
| Classification: This patient does not meet the classification criteria for confirmed perinatal hepatitis C. | ||
*HCV detection tests include a nucleic acid test (NAT) for HCV RNA (including qualitative, quantitative, or genotype testing) or a test indicating the presence of HCV antigen. At present, no HCV antigen tests are approved by the US Food and Drug Administration (FDA). These tests will be acceptable laboratory criteria, equivalent to HCV RNA testing, when an FDA-approved test becomes available.
Appendix D. Hepatitis B Surface Antigen Testing Sequence
All HBsAg testing of patients in the United States should be performed in accordance with Clinical Laboratory Improvement Amendments (CLIA) regulations. The need for additional repeat testing is determined by the signal-to-cutoff (s/c) value during initial HBsAg screening. Confirmatory (e.g., neutralization) testing is required for samples that have that s/c values falling within a specified range for two of three retests, in accordance with the instructions for use provided with the initial HBsAg test assay.
Figure 6-1 illustrates the HBsAg testing algorithm for the Ortho VITROS HBsAg initial assay. For this assay, a s/c value <0.90 from initial HBsAg testing is considered negative, and a s/c value >5.00 is considered positive. S/c values that are <0.90 or >5.00 do not require retesting or additional confirmatory testing, as these are considered clear negative and clear positive, respectively. However, specimens for HBsAg testing ordered on pregnant people might be automatically reflexed to confirmatory testing by some laboratories regardless of the s/c value of the initial test.
A s/c value in the range of 0.90–5.00 on an initial HBsAg test (representing the “gray zone”) indicates the need for retesting, as these could represent samples that are false-positive, low-positive, or have a very high concentration of HBsAg interfering with the test. If the s/c values for two of three repeat tests are less than 1.00, the sample is considered negative. If the s/c values for two of three repeat tests are >5.00, the sample is considered positive. Samples that are considered negative or positive upon repeat testing do not require further testing. Other assays do not use a gray zone or use a different gray zone range to determine the need for repeat testing.
If the s/c values for two of three results are in the range of 1.00–5.00, the sample is considered reactive, and supplemental confirmatory (e.g., neutralization) testing should be performed.
Figure 6-1. Testing algorithm for the Ortho VITROS hepatitis B surface antigen initial assay
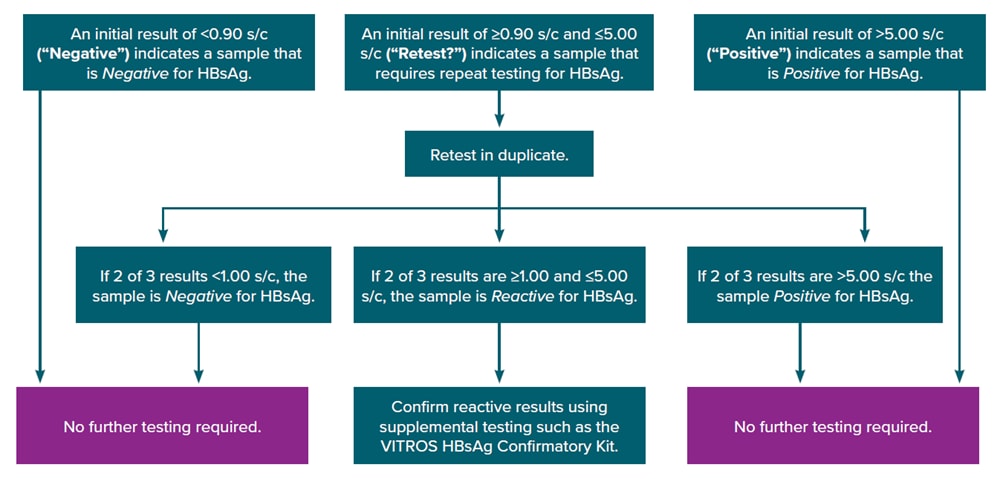
Obtained from https://www.utmb.edu/policies_and_procedures/IHOP/Supporting_Documents/IHOP%20-%2009.13.15%20-%20Serological%20Testing%20for%20Syphilis,%20Hepatitis%20B,%20and%20HIV%20during%20Pregnancy%20and%20Delivery%20(HBsAg_Screening).pdf.
Downloads of this figure: PDF | PPT
In the neutralization test, used as a confirmatory test, specific antibodies are used to bind to HBsAg and when HBsAg is the actual cause of an initial test positive, the amount of HBsAg detected in a repeat assay will be reduced. The final HBsAg qualitative confirmatory result is based on the s/c and % neutralization of the sample as defined in the instructions for use of the confirmatory assay. For example, a confirmed HBsAg positive by the HBsAg Confirmatory Kit by Ortho’s Vitros Immunodiagnostic Products is an undiluted or diluted specimen with s/c >0.8 and neutralization of >50% reduction in signal on assay (122).
The Instructions for Use insert that accompanies the HBsAg confirmatory test kit includes more specific information on testing algorithm and result interpretations. Specimens that should receive HBsAg confirmatory testing, but were not confirmed, or specimens that were negative or indeterminate upon confirmatory HBsAg testing should not be considered positive for HBsAg. If an HD is frequently receiving unconfirmed HBsAg positive results that meet criteria to have been subject to confirmatory testing, the informatics staff at the HD should be consulted and/or the reporting laboratory contacted to clarify testing practices and reporting requirements.