Guidance for Collection, Transport and Submission of Specimens for Ebolavirus Testing
Updated: December 6, 2022
Purpose of this guidance
To provide guidance for hospitals and clinical laboratories on collecting, transporting, and submitting specimens to laboratories to test for ebolaviruses, including Ebola virus (Zaire ebolavirus) and Sudan virus (Sudan ebolavirus). CDC will continue to evaluate new information as it becomes available and will update this guidance as needed.
Scope
This document complements the updated CDC “Guidance for U.S. Hospitals and Clinical Laboratories on Performing Routine Diagnostic Testing for Patients with Suspected Ebola Disease” which provides guidance for clinical laboratories on testing needed for assessment and care of patients for whom Ebola may be a concern, while minimizing risk to laboratory personnel.
This guidance is based on input received from numerous hospital and laboratory directors, infectious disease physicians, CDC Ebola response teams, partners and state health officials.
Key points
- Ebola disease is a rare and deadly disease. In humans, it is caused by an infection with any one of these ebolaviruses: Ebola virus, Sudan virus, Taï Forest virus, Bundibuygo virus. Learn more about Ebola disease and the viruses that cause the disease.
- Early consideration of Ebola disease in the differential diagnosis when appropriate based on clinical and epidemiologic factors is important for providing appropriate and prompt patient care and to prevent the spread of infection. It is important to systematically assess patients through a screening process.
- Clinicians with concerns about Ebola disease in a patient should contact their state, tribal, local, or territorial (STLT) health department immediately. If a diagnosis of Ebola disease is considered, clinical teams should coordinate with STLT public health officials and CDC to ensure appropriate precautions are taken to help prevent potential spread and coordinate care. In the hospital setting, where policies and procedures should be in place to safeguard health care workers, consideration of Ebola disease should not delay diagnostic assessments, laboratory testing, and appropriate care for other, more likely medical conditions.
- CDC’s Viral Special Pathogens Branch (VSPB) is available 24/7 for consultations on Ebola disease or other viral hemorrhagic fevers by calling the CDC Emergency Operations Center at 770-488-7100 and requesting VSPB’s on-call epidemiologist.
- Ebolavirus testing should be performed only after consultation with public health officials (including those at the state or local health department [PDF – 2 pages] and at CDC) to determine that the individual meets criteria for testing as a suspect case of Ebola disease. These criteria include:
- Symptoms including measured (≥100.4°F/38.0°C) or subjective fever, severe headache, fatigue, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage
- An epidemiological risk factor within the 21 days preceding the onset of symptoms
- The United States Occupational Safety and Health Administration (OSHA) developed Bloodborne Pathogens Standard (29 CFR 1910.1030) to reduce the potential exposure of personnel to bloodborne pathogens. All U.S. laboratories handling patient specimens must comply with this standard at all times. Strict adherence is an initial step to protecting personnel.
- Prior to receiving specimens, the laboratory director, safety officer, and/or other responsible persons should already have in place a site-specific risk assessment to minimize risk to personnel from potential exposure from sprays, splashes, or aerosols generated during laboratory activities. Risks should be mitigated by implementing engineering controls, administrative and work practice controls, and use of appropriate personal protective equipment (PPE). A plan for appropriate waste management must also be in place and implemented.
- Ebolaviruses are Department of Transportation (DOT)-classified Category A infectious substances. Specimens that are presumptive positive or from patients with suspected Ebola disease, should be packaged and shipped as Category A infectious substances in accordance with the DOT’s Hazardous Materials Regulations (HMR) Title 49 Code of Federal Regulations (CFR) 173.196
- Consider shipping requirements (e.g., Category A) for specimens being referred for routine diagnostic (non-ebolavirus) testing prior to transferring specimens to another laboratory that would not be aware of the patient’s clinical context.
- Immediately report potential exposures to blood, body fluids, or other infectious materials according to your institution’s policies and procedures.
Specimen collection and testing
If ebolavirus testing is recommended after consultation with STLT public health officials and CDC, a specimen should be collected.
- Wear appropriate personal protective equipment (PPE) when collecting clinical specimens from suspect or confirmed cases. Depending on the patient’s stage of illness, refer to PPE guidance for healthcare workers during management of clinically stable or clinically unstable patients with suspect or confirmed Ebola disease.
- For adults, collect two 4 mL tubes of whole blood in a plastic tube preserved with EDTA. For pediatric patients, collect a minimum of 1 mL whole blood in a pediatric-sized collection tube preserved with EDTA.
- Do not transport or ship specimens in glass containers or in heparinized tubes.
- Do not separate and remove serum or plasma from the primary collection container.
- Public health authorities will determine where ebolavirus testing will occur.
- Presumptive testing for Ebola virus and Sudan virus is available at select LRN reference laboratories throughout the United States. Whole blood specimens should be sent to LRN laboratories on cold packs at 2-8°C (not frozen). Any presumptive positive ebolavirus test result must be confirmed by CDC to inform public health decisions.
- Specimens sent to CDC for testing must be sent on dry ice and arrive at <-20°C.
(Test Order | Submitting Specimens to CDC | Infectious Diseases Laboratories | CDC). Learn more about Packaging and Shipping Clinical Specimens for Ebolavirus Testing at CDC.
- If the specimen test result is negative and the patient’s symptoms have been present for less than three days, a second sample should be collected 72 hours after symptoms and in consultation with public health officials.
Storing clinical specimens for ebolavirus testing
If short-term storage is necessary, keep specimens at 2-8°C for shipping to the LRN laboratory. Specimens must be tested at CDC within 7 days of the date of specimen collection and may be temporarily stored prior to shipment to CDC. Specimens sent to CDC for testing must be sent on dry ice and arrive at <-20°C.
Transporting ebolavirus specimens from site of care through the facility
- Perform a site-specific risk assessment to determine the PPE to be worn during specimen transport within the facility. Required PPE may vary among facilities. Recommendations for PPE include disposable fluid-resistant closed lab coats, disposable gloves, covered legs and closed-toed shoes.
- In compliance with OSHA Bloodborne Pathogens Standard (29 CFR 1910.1030), package sealed specimen containers in a durable, leakproof secondary container.
- Before removing patient specimens from the site of care:
- Plan the route from the patient area to the location where the specimens will be packaged for shipping to avoid high traffic areas.
- Decontaminate the outside of the specimen containers with an approved disinfectant, as described in Interim Guidance for Environmental Infection Control in Hospitals for Ebolavirus.
- For added safety/security, carry specimens in a durable, leakproof secondary container to the laboratory or packaging area by hand. DO NOT use pneumatic tube systems (automated or vacuum specimen delivery system) to transport specimens.
- Once the specimen has been removed from the transport secondary container, disinfect the container.
Note: Recommended disinfectants for use against Ebola virus are found in List L [United States Environmental Protection Agency’s (EPA’s) Disinfectants for Use Against Ebola Virus] and List Q (EPA’s Disinfectants for Emerging Viral Pathogens). These lists of registered disinfectants meet the CDC’s criteria for use against Ebola on hard, non-porous surfaces.
Packaging and transporting specimens for ebolavirus testing outside the facility
- Ebolaviruses are Department of Transportation (DOT)-classified Category A infectious substances. Specimens from patients with suspect or presumptive positive specimens should be packaged and shipped as Category A infectious substances in accordance with the DOT’s Hazardous Materials Regulations (HMR) Title 49 Code of Federal Regulations (CFR) 173.196. Specimens from individuals confirmed to have Ebola disease should be shipped category A and according to select agent regulations.
- All people involved in packaging and shipping infectious substances must be trained and certified in compliance with DOT or the International Air Transport Association (IATA) requirements every two years.
- Do not open collection tubes while packaging specimens to be shipped for ebolavirus testing. Opening tubes will break the vacuum seal and increase the risk of leakage during transport, exposing the packer to the contents.
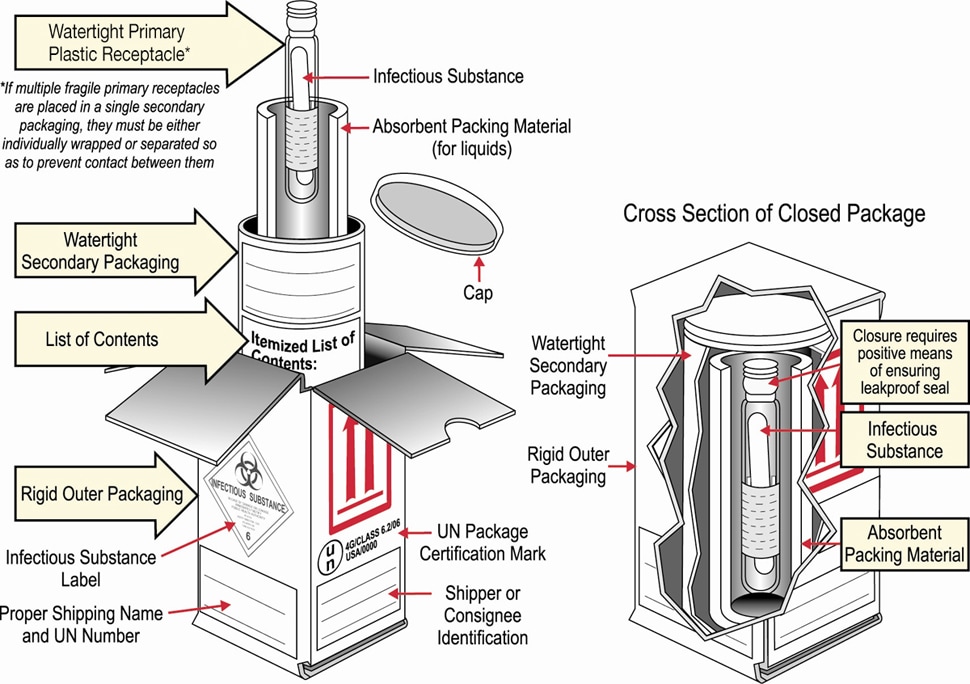
- Package specimens following the triple packaging system, which consists of (1) a primary container (a sealable specimen container) wrapped with absorbent material, (2) a secondary container (watertight, leak-proof), and (3) an outer shipping package that meet Cat A shipping requirements.
- For questions about (packaging) transportation regulations, contact the U.S. DOT Hazardous Materials Information Center at 1-800-467-4922.
- Coordinate with the receiving laboratory on appropriate transport conditions (e.g., cold packs for LRN laboratories or frozen on dry ice for shipment to CDC).
- CDC will not accept specimens without prior consultation and approval. CDC’s Viral Special Pathogens Branch (VSPB) is available 24/7 for consultations by calling the CDC Emergency Operations Center at 770-488-7100 and requesting VSPB’s on-call epidemiologist. Learn more about submitting specimens to CDC for ebolavirus testing.
Occupational health
Immediately report potential exposures to blood, body fluids, or other infectious materials according to your institution’s policies and procedures.
Additional resources and information
- Ebola (Ebola Virus Disease) | CDC
- Ebola outbreak information
- Submitting Specimens to CDC’s Viral Special Pathogens Branch
- Submitting Specimens to CDC Specimen Submission Form
- Infection Prevention and Control Recommendations for Hospitalized Patients with Known or Suspected Ebola in U.S. Hospitals
- Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2008
- Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories [PDF – 105 pages]