Hepatitis A Surveillance Guidance
Background
Hepatitis A is typically a self-limited disease caused by hepatitis A virus (HAV), primarily transmitted fecal-orally after close contact with an infected person or consumption of contaminated food or water (31). Clinical symptoms are indistinguishable from acute hepatitis B and hepatitis C. Hepatitis A is an acute illness and does not result in chronic disease. The United States is considered a low endemicity country with most infections occurring among adults reporting risk behaviors or exposures such as SUD, homelessness, sexual practices resulting in fecal-oral contact, and international travel to hepatitis A-endemic countries (3, 32).
A safe and effective hepatitis A vaccine was licensed in 1995 (33). Prior to vaccine licensure and use, the number of reported hepatitis A cases was around 21,000 annually, and infections were common among children (34, 35). With the widespread adoption of the universal childhood vaccination recommendations in 2006, the overall incidence rate of hepatitis A decreased by 95% across all age groups from 1995 through 2014 (3, 33). However, the incidence rate of hepatitis A increased during 2016–2019 due to widespread person-to-person outbreaks, primarily among PWUD and people experiencing homelessness (3). Increases in hepatitis A have also been reported among MSM (36). A study published in 2020 showed that approximately three-fourths of US-born adults ≥20 years of age were susceptible to hepatitis A during 2007–2016 (37). During 2016–2018, approximately 15,000 hepatitis A cases were reported to CDC, representing a 294% increase compared with 2013–2015 (38). In 2019, the number of hepatitis A cases reported to CDC peaked at 18,846 cases, corresponding to 37,700 estimated infections after adjusting for case under-ascertainment and underreporting (3). The annual number of hepatitis A cases reported to CDC has since declined as more states declared an end to their outbreaks. Disruptions to health care access and health department surveillance capacity during the COVID-19 pandemic may have affected the ability to detect and report all hepatitis A cases.
The purpose of this section is to provide jurisdictional guidance to implement and improve hepatitis A surveillance. Information about reporting requirements, collection of relevant laboratory data, and case investigation is provided. Given that current systems for surveillance differ by jurisdiction, the standards outlined in this document are designed to provide models for best practices based on jurisdictional resources, recognizing that not every jurisdiction is able to meet those standards with available resources.
Uses of Surveillance Data
Hepatitis A surveillance data can be used to inform and improve public health interventions in the following ways:
- Monitoring trends in disease incidence and determining risk behaviors or exposures. Hepatitis A surveillance data should be analyzed at a minimum of weekly by person, place, and time to monitor disease incidence. The proportion of cases reporting specific risk behaviors or exposures should be determined to monitor disease transmission patterns.
- Identifying outbreaks. The identification of a hepatitis A geotemporal cluster or increase in incidence can be an early signal of an outbreak and should prompt further investigation. This investigation should include collection of additional information, including risk behaviors or exposures for person-to-person transmission (e.g., non-injection and injection drug use, homelessness, and sexual and other practices leading to fecal-oral contact) or potential exposures to a common-source (e.g., suspected foods and infected food handler). Surveillance data should be analyzed to determine affected areas (e.g., rates by local jurisdiction or zip code) and groups (e.g., age-specific incidence rates and frequencies of reported risk behaviors or exposures). Prospective surveillance should be conducted to identify additional outbreak cases, identify candidates for post-exposure prophylaxis (if indicated), enhance vaccination efforts for populations at risk, and inform communication and infection control measures. If an outbreak is identified, DVH staff are available to provide consultation.
- Identifying cases among people who might expose others. The identification of a hepatitis A case in someone in a certain occupation (e.g., food handler) or congregate living situations is important because of the potential to expose additional people. This information can facilitate prompt contact tracing and coordination of postexposure prophylaxis.
- Molecular sequencing of viral isolates might help guide response measures. When investigating a possible outbreak, in some instances, collecting sera from patients for diagnosis and molecular characterization (genome sequencing and genotype identification) might provide additional information to guide control efforts and identify outbreaks within outbreaks (e.g., foodborne-related cases during person-to-person outbreak). Public health professionals who need guidance regarding use of nucleic acid testing (NAT) for the investigation of hepatitis A outbreaks should contact CDC’s DVH at hepaoutbreaklab@cdc.gov.
- Assessing missed opportunities for prevention. Patients whose infection source was reported as a household or sexual contact with suspected or confirmed hepatitis A should be investigated to determine if the patient received post-exposure prophylaxis when the source case was identified. In addition, surveillance data can be used to provide information about people at high risk for infection to provide education and awareness about the importance of vaccinating populations as recommended by the Advisory Committee on Immunization Practices (ACIP).
- Assessing the impact of vaccination programs. Age-specific incidence rates for the priority groups and the community as a whole can be compared to historical rates for the same age groups to assess the impact of routine vaccination programs.
Cases and Clusters of Potential Public Health Importance
Jurisdictions should review and analyze hepatitis A data regularly to identify cases and clusters of hepatitis A that merit further investigation. Ideally, all cases of reported hepatitis A should be investigated. In jurisdictions with limited resources, cases and clusters should be prioritized for investigation in accordance with the degree of public health importance. The following are examples of cases that are high priority for further follow-up:
- Cases in people who are in higher risk groups (e.g., PWUD, people experiencing homelessness) or who live in congregate settings (e.g., shelters, correctional facilities) to assure that interventions to prevent further spread are implemented in a timely fashion
- Cases who were previously vaccinated to characterize possible vaccine failures (see Section 1.10).
- Cases of hepatitis A in people born after 2005 to distinguish between failure of vaccine and failure to vaccinate
- Cases without common risk behaviors or exposures
- Two or more cases among patrons at the same store or food service establishment
Interpretation of Laboratory Test Results
Immunoglobulin M antibody to hepatitis A virus (anti-HAV IgM) and viremia identified by HAV NAT using polymerase chain reaction (PCR) can detect recent or current acute infection with HAV. A description of hepatitis A laboratory markers can be found in Appendix B. Figure 2-1 illustrates the typical serologic course of HAV infection and recovery.
Figure 2-1. Typical serologic course of hepatitis A virus infection and recovery
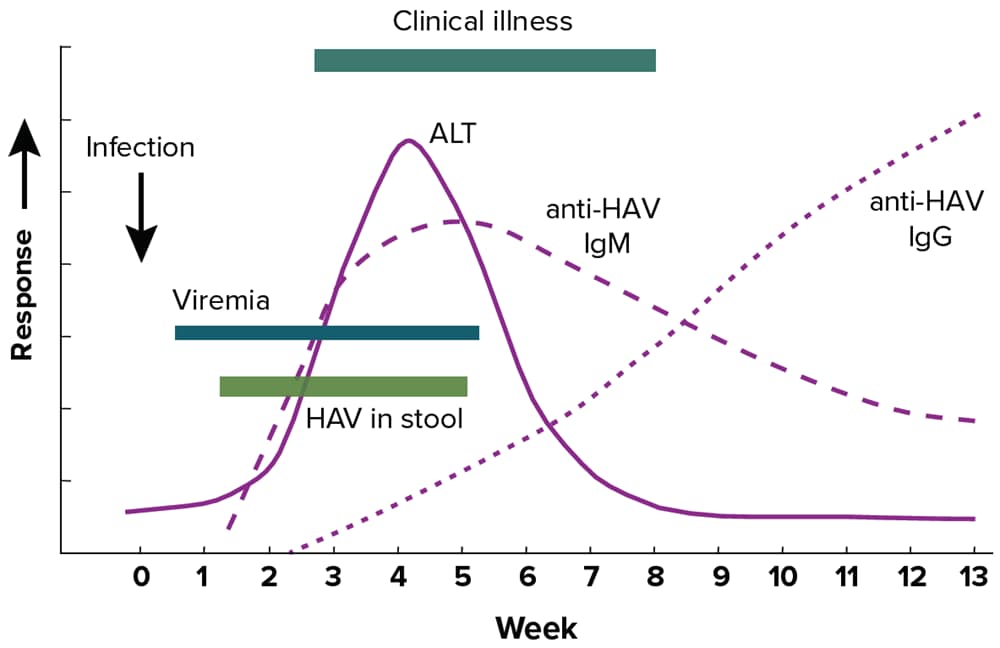
Figure obtained from https://www.cdc.gov/mmwr/pdf/rr/rr5304.pdf.
Downloads of this figure: PDF | PPT
Caution should be exercised when interpreting a positive anti-HAV IgM laboratory result, as positive tests can occur in people >1 year after infection and false-positive tests can also occur in those without clinical or epidemiologic evidence of recent infection (39). A person with a positive anti-HAV IgM result may also be positive for anti-HAV IgG and total anti-HAV. Because of the risk of misinterpreting positive results, anti-HAV IgM testing should be limited to people with clinical presentation of hepatitis who are suspected of having hepatitis A. Anti-HAV IgM testing should not be used as a screening tool or as part of testing panels in the workup of abnormal liver function tests. Some conditions might cause cross-reactivity with anti-HAV IgM tests, including infection with the Epstein-Barr virus (40) and hepatitis C virus (41). Furthermore, some infected people might initially test negative for anti-HAV IgM during the first few days of symptoms (42). If there is high clinical suspicion of hepatitis A in a person who has a negative test for anti-HAV IgM early in their clinical course, repeat testing may be indicated (42). One study found that the optimal time for repeat testing is at least 2 days after ALT levels have peaked (42).
Table 2-1 interprets the combinations of total anti-HAV and anti-HAV IgM laboratory results frequently available in viral hepatitis test panels, following the biomarker changes over the course of infection as shown in Figure 2-1. If HAV RNA testing is performed, a detectable HAV RNA level indicates the presence of infection.
Table 2-1. Interpretation of hepatitis A laboratory results
| Total anti-HAV | Anti-HAV IgM | Interpretation* |
|---|---|---|
| Positive | Positive | Current infection, recent infection, or recent vaccination. |
| Positive | Not done | Previous infection or current infection; cannot differentiate recent from remote infection or prior vaccination. |
| Positive | Negative | Previous infection or vaccination. |
| Negative | Negative | Not infected (i.e., susceptible). |
| Not done or negative | Positive | Current infection or false-positivity/cross-reactivity. |
*Ingestion of high levels of biotin can significantly interfere with certain commonly used biotinylated immunoassays, such as those used to detect anti-HAV, and cause false-positive or false-negative laboratory test results. Currently, the US Food and Drug Administration (FDA) is investigating thresholds associated with false-positive and false-negative tests. This section will be updated as more information becomes available. Reference: https://www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication.
Downloads of this table: PDF | PPT
Recommended Reportable Laboratory Markers
To aid in hepatitis A surveillance, the following laboratory markers should be reported to public health agencies:
- Positive anti-HAV IgM;
- Positive/detectable HAV RNA (including qualitative, quantitative, or genotype testing); and
- All concurrent ALT and total bilirubin results reported with positive hepatitis A laboratory results, which can also be helpful in classifying hepatitis A cases that do not have an HAV RNA laboratory result.
Surveillance Case Definition
Table 2-2 specifies the surveillance case definition for hepatitis A, adopted by CSTE and CDC in 2019. This definition should be used for hepatitis A case classification and national notification (12, 43). See Appendix C for classification scenarios of cases of hepatitis A.
Table 2-2. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for hepatitis A, 2019
| Criteria Type | Criteria |
|---|---|
| Clinical |
|
| Laboratory* |
|
| Epidemiologic Linkage |
|
| Case Status | Classification |
| Confirmed* |
|
†And not otherwise ruled out by anti-HAV IgM or NAAT for HAV RNA testing performed in a public health laboratory.
Downloads of this table: PDF | PPT
Up to 10% of people with hepatitis A might experience a relapse of symptoms during the 6 months after acute illness. Cases of relapsing hepatitis A should not be enumerated as new cases. In addition, a case should not be counted as a hepatitis A case if there is an alternate, more likely diagnosis.
Case Ascertainment
The primary method of ascertaining cases is by reviewing reports from clinical laboratories, health care facilities, and health care providers. All states should have rules or regulations requiring that these facilities report evidence of hepatitis A to public health agencies. See Section 1.6. and Section 2.5. for information on the recommended reporting requirements for hepatitis A.
Laboratory Reporting
All states require clinical laboratories to report hepatitis A laboratory markers, such as positive anti-HAV IgM and positive HAV RNA results.
Health Care Facility and Provider Reporting
All states require health care facilities and providers to report hepatitis A diagnoses.
Additional sources that will facilitate case ascertainment and case characterization include medical records, hospital discharge databases, and death certificates. Section 5.4 describes the usefulness of select data sources in supplementing case ascertainment.
Figure 2-2 illustrates one approach for hepatitis A case ascertainment and classification. Specific procedures might vary by jurisdiction, but should generally follow the scheme outlined in Figure 2-2, in accordance with the CDC/CSTE Position Statement for the 2019 hepatitis A case definition (12, 43). See Appendix C for case classification scenarios for hepatitis A.
Figure 2-2. Process for hepatitis A case ascertainment and classification
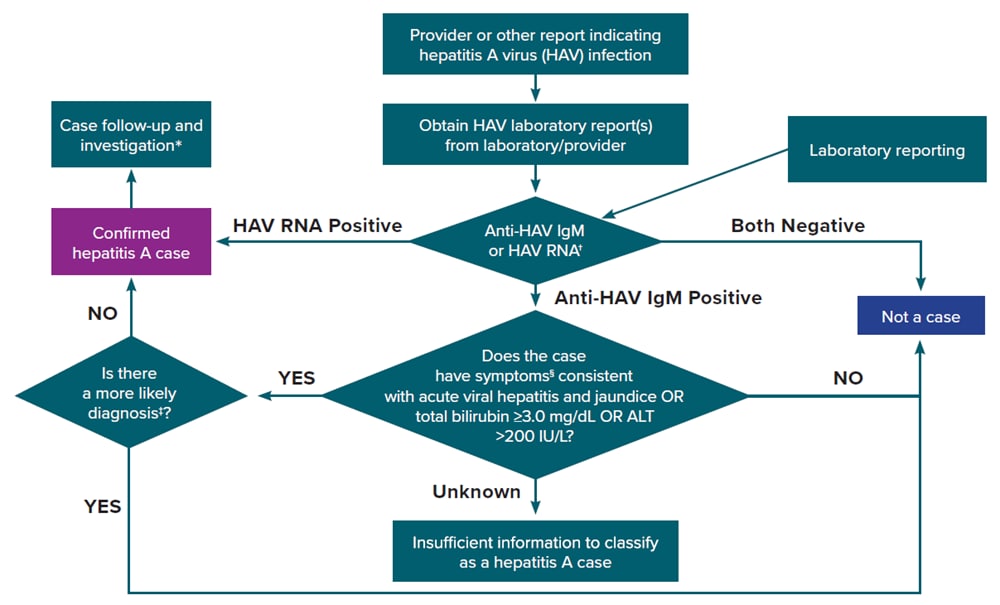
†Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling, as appropriate
‡May include evidence of acute liver injury from infectious, autoimmune, metabolic, drug or toxin exposure, neoplastic, circulatory or thromboembolic, or idiopathic causes.
§Clinical symptoms include fever, headache, malaise, anorexia, nausea, vomiting, diarrhea, abdominal pain, or dark urine.
Downloads of this figure: PDF | PPT
Case Investigation
Reports from laboratories, health care providers, and other data sources indicative of hepatitis A should be submitted to HDs (as specified by local regulations) and investigated as soon as possible to ensure adequate time to implement preventive measures (e.g., vaccination of contacts). Suspected cases of hepatitis A should be reported with appropriate laboratory results and clinical information (Table 2-2). For general information on conducting viral hepatitis case investigations, see Section 1.10. The following is a description of the follow-up activities that should be conducted for reported hepatitis A cases:
Information from the Laboratory
Positive anti-HAV IgM and positive/detectable HAV RNA laboratory results should be reported to the HD and investigated immediately. Other laboratory information that can assist with case classification includes ALT and total bilirubin levels.
Information from the Provider or Medical Records
The following information might be available from medical records to confirm the diagnosis, inform case classification, and determine public health priority.
- Diagnostic test results. Hepatitis A laboratory markers (e.g., positive anti-HAV IgM and positive/detectable HAV RNA) should be reportable to the HD. If additional laboratory testing (e.g., for ALT and total bilirubin levels) is needed to classify the case, HD staff will work with the provider to obtain these test results.
- Clinical features. Includes reason for testing, illness onset date, clinical signs and symptoms (including the presence of jaundice), coinfections, hospitalization status and date of death, and whether hepatitis A or an alternate diagnosis is suspected.
- Demographic information. Includes name, date of birth, sex at birth, current gender, race, ethnicity, and residential address (including zip code).
- Risk behaviors or exposures. Includes non-injection and injection drug use, experience of unstable housing/homelessness, high-risk sexual practices, occupation, international travel history, international adoption history, and household or sexual contact with someone with a confirmed or suspected case of hepatitis A.
- Patients who deny known risk behaviors or exposures for infection can be interviewed with a supplemental food history questionnaire.
- At the earliest possible point, information regarding whether the patient is in a sensitive occupation (e.g., food handler) or an attendee or resident of a congregate setting should be obtained.
- Occupation. While no documented evidence indicates that food handlers or health care workers are at higher risk for infection than people in other occupations, jurisdictions routinely obtain this information to inform contact tracing. Special attention should be given to the job duties of people in sensitive occupations, including whether the patient was symptomatic while at work, which symptoms (if any) were experienced while at work, and the patient’s work schedule during their infectious period. Food handlers should be restricted from working in a food handling capacity while infectious, and patrons from food service establishments or health care providers should be notified as appropriate (44).
- Vaccination information. Hepatitis A vaccination has been recommended for infants since 2006 in all US states. Depending on age of the HAV-infected person, some cases of hepatitis A should have been vaccinated in childhood, whereas others should have been vaccinated as adults because they met specific risk criteria. Though rare, recent vaccination might result in transient anti-HAV IgM positivity. Obtaining vaccination history can be done via the patient’s provider or state immunization registries and is useful in identifying vaccine failure or transient anti-HAV IgM positivity.
Information from the Patient
Resources permitting, all patients with hepatitis A should be contacted for an interview using the jurisdiction-specific case investigation form. At a minimum, all patients who are classified as “confirmed” per the CDC/CSTE case definition should be interviewed. The patient interview should ideally include the following components:
- Epidemiologic link. For all laboratory-confirmed cases of hepatitis A, obtain information on contacts where exposure occurred 15–50 days prior to the onset of symptoms and investigate whether contacts met the clinical criteria.
- Risk behaviors or exposures. To determine the most likely mode of transmission, ask patients about behaviors and exposures during the 15–50 days prior to illness onset. Patients who deny other risks for infection should be interviewed with a supplemental food history questionnaire.
- Education and referral for follow-up. Assess whether the patient requires education or other medical follow-up services, including hepatitis B vaccination, as appropriate. People with hepatitis A should be counseled on how to prevent transmission to others.
- Identification of contacts requiring post-exposure prophylaxis. If resources allow, identify contacts and coordinate referral for post-exposure prophylaxis if contact occurred within 14 days. Information regarding hepatitis A vaccination and post-exposure prophylaxis can be found on the Hepatitis A ACIP Vaccine Recommendations website.
Special Considerations when Investigating Certain Populations or Settings at Risk for Rapid Disease Transmission
Certain populations and settings are associated with increased risk for rapid transmission of hepatitis A. Considerations when investigating hepatitis A cases among people experiencing homelessness, PWUD, people engaging in high-risk sexual practices, and people in correctional facilities are provided in Section 1.10.
Outbreak Reporting and Notification
All hepatitis A outbreaks should be reported to the appropriate local authorities for further investigation within the timeframe each jurisdiction has specified. The reporting timeframe to local authorities varies by jurisdiction. Notification to CDC is done through NNDSS and by contacting viralhepatitisoutbreak@cdc.gov, as indicated in CDC-RFA-PS21-2103. See Section 5.3 for guidance on reporting outbreak source to NNDSS.
Case Reporting and National Notification
Cases of hepatitis A should be reported to HDs as specified by state, territorial, and local regulations. Hepatitis A is a nationally notifiable condition (9). Hepatitis A cases are identified using an event code (Table 1-2). Data are sent weekly or more frequently, depending on the infrastructure of the jurisdiction sending the data. Cases might be re-classified or removed as needed after the initial transmission to CDC, if the changes occur before surveillance data are finalized each year.