Flu Vaccination Coverage, United States, 2011-12 Influenza Season
- On This Page
Data Sources
National Immunization Survey (NIS) and Behavioral Risk Factor Surveillance System (BRFSS).
Introduction
Flu vaccination is the most effective strategy to prevent people from getting the flu and potentially serious flu-related complications (1). For this reason, the Advisory Committee on Immunization Practices (ACIP) recommends flu vaccination for everyone 6 months and older (2).
To estimate monthly cumulative flu vaccination coverage from August 2011 through May 2012, CDC analyzed the National Immunization Survey (NIS) data for children 6 months–17 years and the Behavioral Risk Factor Surveillance System (BRFSS) data for adults ≥18 years. Coverage estimates are presented here by age group, race/ethnicity, and month of vaccination with additional information for adults with medical conditions (e.g., asthma, diabetes, or heart disease) that put them at high risk for flu-related complications.
Monthly cumulative estimates for each state, each Health and Human Services region, and the United States by age and racial/ethnic groups are provided in FluVaxView as interactive maps, figures, and tables.
Key Findings
- Flu vaccination coverage among children did not significantly change for the 2011–12 season compared to the 2010–11 season.
- Flu vaccination coverage for adults decreased (by 1.7 percentage points) for the 2011-12 season compared to the 2010-11 season.
- Changes in estimates of adult flu vaccination coverage from seasons prior to 2011-12 may be partially due to changes in BRFSS methodology introduced in 2011-12. The BRFSS sample was expanded to include households with only cellular telephones in addition to the original sample of households with landline telephones creating a dual sample frame and requiring a change in weighting methods (3).
- State variability in adult and child influenza coverage continues to be large.
Who Was Vaccinated?
Coverage by Age Group
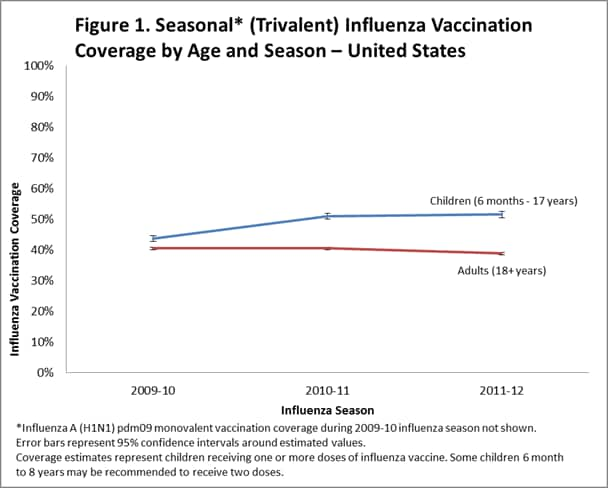
- Among all persons ≥6 months, national influenza vaccination coverage August 2011 through May 2012 was 41.8%, 1.2 percentage-points lower than 2010–11 seasonal coverage.
- State-specific estimates ranged from 32.6% (Nevada) to 51.1% (South Dakota).
- Among all age groups, the estimated national coverage varied widely.
- Coverage for children decreased with increasing age:
- 6-23 months: 74.6%
- 2-4 years: 63.3%
- 5-12 years: 54.2%
- 13-17 years: 33.7%
- Coverage for adults increased with increasing age:
- 18–49 years: 28.6%
- 50–64 years: 42.7%
- ≥65 years: 64.9%
- Coverage for children decreased with increasing age:
| Age Group | Unweighted Sample Size | %§ | 95% CI¶ |
|---|---|---|---|
| ≥6 months | 463,754 | 41.8 | ±0.4 |
| 6 months−17 years | 96,254 | 51.5 | ±1.0 |
| ≥18 years | 367,500 | 38.8 | ±0.4 |
Children (6 months to 17 years)
- Among children 6 months–17 years, national coverage with one or more doses was 51.5%, similar to 2010–11 (51.0%) coverage.
- State-specific coverage for children ranged from 38.8% (Alaska) to 73.8% (Rhode Island).
| Age Group | Unweighted Sample Size | %§ | 95% CI¶ |
|---|---|---|---|
| 6 months−17 years | 96,254 | 51.5 | ±1.0 |
| 6 months−4 years | 25,206 | 67.6 | ±1.7 |
| 6−23 months | 9,115 | 74.6 | ±2.5 |
| 2−4 years | 16,091 | 63.3 | ±2.3 |
| 5−12 years | 40,584 | 54.2 | ±1.4 |
| 13−17 years | 30,464 | 33.7 | ±1.6 |
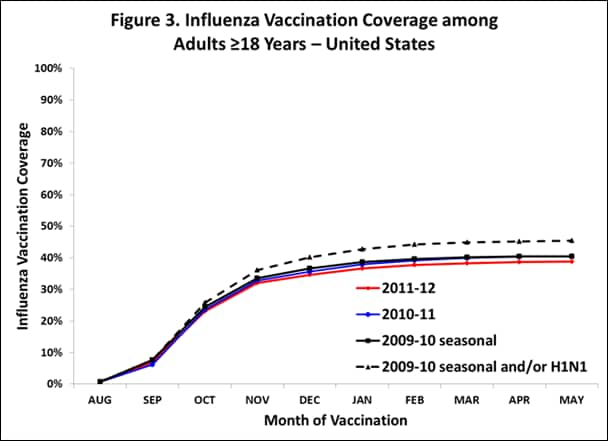
- Among adults ≥18 years, national coverage was 38.8%, 1.7 percentage points lower than 2010-11 coverage (40.5%).
- State-specific coverage for adults ranged from 28.3% (Nevada) to 48.9% (South Dakota).
- Among adults 18–49 years, estimated national coverage for the 2011–12 season (28.6%) was lower than 2010–11 seasonal coverage (30.5%).
- State-specific coverage ranged from 21.8% (Nevada) to 41.2% (South Dakota).
- Among adults 50–64 years, estimated national coverage (42.7%) for the 2011–12 season was lower than 2010–11 seasonal coverage (44.5%), and 2009–10 coverage (45.0%).
- State-specific coverage ranged from 27.3% (Nevada) to 52.4% (Tennessee).
- Among adults ≥65 years, estimated national coverage for the 2011–12 season (64.9%) was 1.7 percentage-points lower than 2010–11 seasonal coverage (66.6%), and 4.7 percentage-points lower than 2009–10 coverage (69.6%).
- State-specific coverage ranged from 49.5% (Alaska) to 75.9% (Iowa).
- Among adults 18–49 years with at least one selected high-risk medical condition measured by BRFSS (asthma, diabetes or heart disease), estimated national coverage was 36.8%, similar to 2010–11 seasonal coverage (39.0%) and 2009–10 (trivalent) coverage (38.2%).
- State-specific coverage varied widely, ranging from 25.2% (Nevada) to 53.1% (Hawaii).
| Age Group | Unweighted Sample Size | %§ | 95% CI¶ |
|---|---|---|---|
| ≥18 years | 367,500 | 38.8 | ±0.4 |
| 18−64 years | 250,326 | 33.1 | ±0.6 |
| 18−64 years at high risk|| | 49,403 | 45.2 | ±1.2 |
| 18−49 years | 131,452 | 28.6 | ±0.6 |
| 18−49 years at high risk|| | 18,444 | 36.8 | ±2.0 |
| 50−64 years | 118,874 | 42.7 | ±0.8 |
| ≥65 years | 117,174 | 64.9 | ±0.8 |
Coverage by Sex
Children (6 months to 17 years)
- For children, coverage was similar between sexes.
- Among adults 18–64 years, the estimated national coverage was 6.9 percentage-points higher for females compared with males, 36.5% and 29.6%, respectively.
- Among high-risk adults 18–64 years, coverage was 4.6 percentage-points higher for females compared to males, 47.3% and 42.7%, respectively.
- For adults 65 years and older, coverage was similar between sexes.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Age Group | Unweighted Sample Size | %§ | 95% CI¶ | Unweighted Sample Size | %§ | 95% CI¶ |
| All ages | ||||||
| ≥6 months | 196,704 | 39.4 | ±0.7 | 267,050 | 44.1 | ±0.6 |
| Children | ||||||
| 6 months−17 years | 49,566 | 51.7 | ±1.3 | 46,688 | 51.4 | ±1.4 |
| 6 months−4 years | 12,786 | 68.2 | ±2.3 | 12,420 | 66.9 | ±2.6 |
| 6−23 months | 4,553 | 73.3 | ±3.6 | 4,562 | 75.9 | ±3.4 |
| 2−4 years | 8,233 | 65.2 | ±2.9 | 7,858 | 61.4 | ±3.5 |
| 5−12 years | 20,900 | 54.6 | ±1.8 | 19,684 | 53.7 | ±2.2 |
| 13−17 years | 15,880 | 33.1 | ±2.2 | 14,584 | 34.4 | ±2.3 |
| Adults | ||||||
| ≥18 years | 147,138 | 35.4 | ±0.8 | 220,362 | 42.0 | ±0.6 |
| 18−64 years | 104,442 | 29.6 | ±0.8 | 145,884 | 36.5 | ±0.6 |
| 18−64 years at high risk|| | 19,450 | 42.7 | ±2.0 | 29,953 | 47.3 | ±1.6 |
| 18−49 years | 56,224 | 25.6 | ±1.0 | 75,228 | 31.8 | ±0.8 |
| 18−49 years at high risk|| | 6,619 | 34.0 | ±3.3 | 11,825 | 39.0 | ±2.4 |
| 50−64 years | 48,218 | 39.1 | ±1.2 | 70,656 | 46.0 | ±1.0 |
| ≥65 years | 42,696 | 64.8 | ±1.2 | 74,478 | 65.0 | ±0.8 |
Coverage by Race/Ethnicity
- Among persons ≥6 months, coverage for non-Hispanic whites (43.1%) was higher than that of non-Hispanic blacks (39.0%), Hispanics (39.3%), and other and multiple races/ethnicities (37.5%), and was similar to coverage among Asians (41.1%), and American Indians/Alaska Natives (AIAN) (43.9%).
| Race/Ethnicity | Unweighted Sample Size | %§ | 95% CI¶ |
|---|---|---|---|
| White only, non-Hispanic | 349,760 | 43.1 | ±0.4 |
| Black only, non-Hispanic | 41,070 | 39.0 | ±1.4 |
| Hispanic | 38,077 | 39.3 | ±1.3 |
| Other, non-Hispanic (Total) | 30,971 | 40.0 | ±1.7 |
| Asian | 10,031 | 41.1 | ±3.0 |
| American Indian Alaska Native | 6,127 | 43.9 | ±4.4 |
| Other and multiple race** | 14,813 | 37.5 | ±2.3 |
Children (6 months to 17 years)
- Among children 6 months–17 years, coverage among non-Hispanic Asians (58.2%), blacks (53.7%) and Hispanics (59.5%) was higher than non-Hispanic whites (47.6%).
- Coverage for AIAN (52.3%) and other or multiple races/ethnicities (50.0%) was similar to non-Hispanic whites.
- Compared to the 2010-11 season, coverage in the 2011-12 season decreased 0.9 percentage points for non-Hispanic whites, but increased 4.4 and 2.9 percentage points for Hispanics (55.1%) and non-Hispanic blacks (50.8%), respectively.
Race/EthnicityUnweighted Sample Size%§95% CI¶
| White only, non-Hispanic | 60,270 | 47.6 | ±1.0 |
|---|---|---|---|
| Black only, non-Hispanic | 10,453 | 53.7 | ±2.9 |
| Hispanic | 15,665 | 59.5 | ±2.5 |
| Other, non-Hispanic (Total)¶ | 9,866 | 53.6 | ±2.8 |
| Asian | 3,510 | 58.2 | ±4.7 |
| American Indian Alaska Native | 1,326 | 52.3 | ±7.2 |
| Other and multiple race** | 5,030 | 50.0 | ±4.0 |
For additional race/ethnicity estimates by age group, please see attached tables [12 KB] Adults (18 years and older)
- Among adults ≥18 years, coverage for non-Hispanic whites (41.9%) was higher than coverage for all other racial/ethnic groups except AIAN (42.6%); Hispanics (29.4%); blacks (32.7%); other and multiple races/ethnicities (33.9%); and Asians (37.3%).
- Adult coverage for non-Hispanic blacks, Asians, AIAN, and other and multiple races/ethnicities in 2011–12 (32.7%, 37.3%, 42.6%, and 33.9%, respectively) was comparable to 2010–11 seasonal coverage (34.2%, 38.2%, 39.0%, and 37.4%, respectively), but lower by 1.3 and 2.9 percentage-points, respectively, in 2011–12 for non-Hispanic whites (41.9%) and Hispanics (29.4%).
| Race/Ethnicity | Unweighted Sample Size | %§ | 95% CI¶ |
|---|---|---|---|
| White only, non-Hispanic | 289,490 | 41.9 | ±0.4 |
| Black only, non-Hispanic | 30,617 | 32.7 | ±1.6 |
| Hispanic | 22,412 | 29.4 | ±1.6 |
| Other, non-Hispanic (Total) | 21,105 | 36.7 | ±2.0 |
| Asian | 6,521 | 37.3 | ±3.5 |
| American Indian Alaska Native | 4,801 | 42.6 | ±4.9 |
| Other and multiple race** | 9,783 | 33.9 | ±2.7 |
For additional race/ethnicity estimates by age group, please see attached tables [12 KB] Top of Page
Coverage by Month
Children (6 months to 17 years)
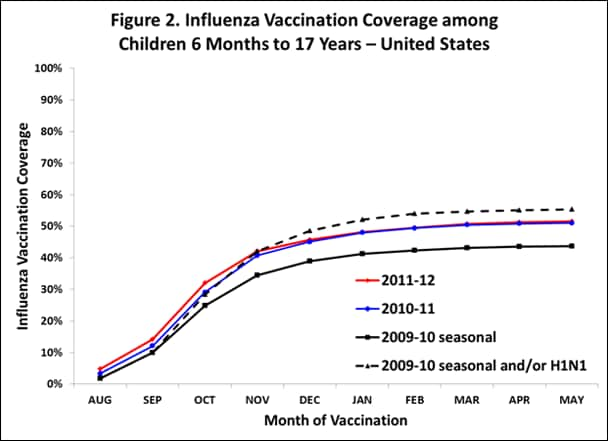
- Among children 6 months–17 years, monthly coverage by the end of May was comparable for the 2011–12 season compared to 2010–11 monthly seasonal coverage.
- Among children 6 months–17 years, cumulative monthly coverage was one to three percentage points higher early in the 2011-12 season compared to 2010-11 (August-November), but the uptake curves were similar the rest of the season (December-June) (Figure 2).
Estimated Numbers of Persons Vaccinated
Based on the NIS and BRFSS, the estimated number of persons reporting receipt of one or more seasonal influenza vaccinations was 37.3 million (95% Confidence Interval [CI] 36.6 – 38.0 million) children (6 months-17 years) and 90.6 million (95% CI 89.6 – 91.5 million) adults (≥18 years), for an estimated 127.9 million (95% CI 126.6 – 129.1 million) people vaccinated against seasonal influenza during August 2011 through May 2012 among the civilian, non-institutionalized U.S. population. The actual number of influenza vaccination doses distributed during the 2011-12 season was 132.8 million. Top of Page
What Can Be Done?
Overall, for all persons 6 months and older, estimated flu vaccination coverage was slightly lower in 2011-12 (41.8%) compared to the prior season (43.0%). For all groups, flu vaccination coverage for the 2011-12 season remained well below the Healthy People 2020 targets of 80% for persons 6 months–64 years and 90% for adults 65 years and older and adults 18–64 years with medical conditions that put them at high risk for complications from flu (4). Racial/ethnic disparities in influenza vaccination among adults remained and considerable variation in coverage by state was observed. Continued efforts are needed to ensure those at highest risk of flu complications (i.e., elderly, young children, and persons with chronic health conditions) are vaccinated each year.
Since flu vaccination is the most effective prevention strategy against the flu and serious flu-related complications, immunization programs are encouraged to utilize available strategies to increase coverage such as:
- Expanding access through non-traditional settings, e.g., pharmacy, workplace, and school venues for vaccination to reach individuals who may not visit a traditional provider during the flu season.
- Improving use of evidence-based practices at medical sites (e.g., standing orders, reminder/recall notification, provider recommendation) to ensure that all those who visit a provider during the flu season receive a vaccination recommendation and offer (5,6).
- Utilizing immunization information systems, also known as registries, at the point of clinical care and at the population level to guide clinical and public health vaccination decisions.
These and other strategies are described in the Community Guide for Preventive Services (6).
To help immunization programs improve vaccination coverage and assess strategies implemented to reach those at highest risk for flu complications, flu vaccination monitoring and assessments systems must be constantly improved. The recent changes in BRFSS methods to a dual frame survey by adding a sample of cell phone only households to the traditional sample of landline households improved the representativeness of the sample compared to the general population where cell phone use is increasing. To monitor the effect of this change on flu vaccination coverage estimates we compared the BRFSS adult flu vaccination coverage estimates to estimates from the National Health Interview Survey (NHIS), a household in-person survey. Using the available NHIS data which provided estimates of vaccination coverage for the 2011-12 influenza season through November, no decrease in coverage was found compared to the previous 2010-11 season. Also, estimates from the March National Flu Survey indicated a slight increase in influenza vaccination coverage among adults from the 2010-11 to 2011-12 seasons (March Flu Vaccination Coverage). This leads us to believe that the slight decrease in adult coverage found in this report based on the BRFSS data is likely due to the methodological changes to the survey. When data from BRFSS are available for the 2012-13 influenza season, the trend in adult influenza vaccination coverage will be more fully understood. Regardless of the slight impact that the methodological changes might have had on the 2011-12 season estimates, influenza vaccination coverage remains well below the national targets indicating that more work is needed to protect people from influenza. Top of Page
Data Source and Methods
CDC analyzed NIS and BRFSS data collected September 2011 through June 2012 from all 50 states and the District of Columbia to estimate national and state level influenza vaccination coverage for the 2011–12 influenza season. These findings were compared to 2010-11 influenza season estimates.
NIS is an ongoing, national landline list-assisted random-digit-dialed telephone survey of households with children who are 19–35 months or 13–17 years (NIS-Teen) at the time of interview. For children 6–18 months and 3–12 years identified during screening households for NIS and NIS-Teen, a short influenza vaccination module was added. A supplemental cellular phone sample was conducted as part of the NIS. Respondents ≥18 years were asked if their children had received a flu vaccination since July 1, 2011, and, if so, in which month and year. If the child received a vaccination, respondents were asked how many vaccinations. The NIS Council of American Survey and Research Organizations (CASRO) response rates across three quarters of data collection ranged from 51.8%–57.3% for landline and 19.9%–30.3% for cellular telephones. The CASRO response rate is the product of three other rates: 1) the resolution rate, which is the proportion of telephone numbers that can be identified as either for a business or residence; 2) the screening rate, which is the proportion of qualified households that complete the screening process; and 3) the cooperation rate, which is the proportion of contacted eligible households for which a completed interview is obtained.
BRFSS is an on-going state-based monthly telephone survey which collects information on health conditions and risk behaviors from ~400,000 randomly selected persons ≥18 years among the non-institutionalized, U.S. civilian population. BRFSS respondents were asked if they had received a ‘flu’ vaccine in the past 12 months, and if so, in which month and year. The median state CASRO BRFSS response rate was 49.2% for September-December 2011 and 47.5% for January-June 2012. Changes made to BRFSS in 2011-12 included adding persons in households with only cellular service and improvements to weighting procedures (3).
For calculation of influenza vaccination coverage estimates from both surveys, Kaplan-Meier survival analysis was used to determine the cumulative influenza vaccination coverage (≥1 dose) during August 2011 through May 2012 using monthly interview data collected September 2011 through June 2012. NIS data were used to estimate coverage for children 6 months–17 years and BRFSS data were used to estimate coverage for adults ≥18 years. Coverage estimates for all persons ≥6 months were determined using combined state-level monthly estimates weighted by the age-specific populations of each state (7). For 14.9% of vaccinated NIS-Flu participants and 7.1% of vaccinated BRFSS participants, month of vaccination was missing and was imputed from donor pools matched for week of interview, age group, state of residence, and race/ethnicity. Results from both surveys were weighted and analyzed with SAS and SUDAAN statistical software to account for the complex survey design. Differences between groups and between 2010-11 and 2011-12 seasons were determined using t-tests with significance at p<0.05. Top of Page
Limitations
The estimates in this report are subject to the following limitations. First, influenza vaccination status was based on self or parental report, not validated with medical records, and, thus, is subject to respondent recall bias. Second, response rates for both surveys were low and nonresponse bias may remain even after weighting adjustments. Third, combining NIS and BRFSS estimates allowed estimation of coverage for all persons ≥6 months; however, differences in survey methodology (e.g., different sampling frame, survey design, exact survey question wording, response rates and weighting) may result in different levels of bias that are averaged for this group. Fourth, the number of persons vaccinated might be overestimated, as previous estimates have resulted in higher numbers vaccinated than doses distributed (8). Finally, some age-by-state-specific estimates in the accompanying interactive reports may be unreliable due to small sample size. Estimates flagged as potentially unreliable should be interpreted with caution.
Authors
Anne F. McIntyre, PhD, MPH; Amparo G. Gonzalez-Feliciano, MPH; Tammy A. Santibanez, PhD; Leah N. Bryan, MPH; Stacie M. Greby, DVM, MPH; Bradley B. Biggers, MPH; James A. Singleton, PhD Top of Page
Related Links
- National Immunization Survey (NIS):
- https://www.cdc.gov/nchs/nis.htm
- https://www.cdc.gov/vaccines/stats-surv/imz-coverage.htm
- Behavioral Risk Factor Surveillance System (BRFSS):
- https://www.cdc.gov/brfss/
- https://www.cdc.gov/vaccines/stats-surv/brfss/default.htm
- Influenza vaccination coverage reports:
- Influenza general information:
References
- Cox NJ and Subbarao K. Influenza. Lancet 1999;354:1277-82.
- CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59(No. RR-8).
- CDC. Methodologic changes in the Behavioral Risk Factor Surveillance System in 2011 and potential effects on prevalence estimates. MMWR 2012;61(22):410-13.
- US Department of Health and Human Services. Healthy People 2020. Available at http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23. Accessed September 12, 2012.
- Interim results: state-specific seasonal influenza vaccination coverage – United States, August 2010-February 2011. MMWR 2011; 60(22):737-743.
- Guide to Community Preventive Services. Available at http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed September 10, 2012.
- Furlow-Parmley C, Singleton JA, Bardenheier B, Bryan L. Combining estimates from two surveys: an example from monitoring 2009 influenza A(H1N1) pandemic vaccination. Statist Med DOI: 10.1002/sim.5333
- CDC. Interim results: state-specific seasonal influenza vaccination coverage―United States, August 2009–January 2010. MMWR 2010;59:477–84.
Footnotes
* Weighted coverage estimates are for persons interviewed September 2011 through June 2012 who reported being vaccinated August 2011 through May 2012. † Excludes U.S. territories. § Month of vaccination was imputed for respondents with missing month of vaccination data. Percentages are weighted to the non-institutionalized, U.S. civilian population. ¶ Confidence interval half-widths. || Selected high risk (HR) conditions; includes people with asthma, diabetes or heart disease.
** Includes Native Hawaiian or other Pacific Islander, multiracial, and other races. Top of Page