FluView Summary ending on July 29, 2023
Updated August 4, 2023

2022-2023 Influenza Season
Week 30 ending July 29, 2023
All data in this report are preliminary and may change as more reports are received.
A description of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available on the surveillance methods page.
Additional information on the current and previous influenza seasons for each surveillance component is available on FluView Interactive.
U.S. Virologic Surveillance
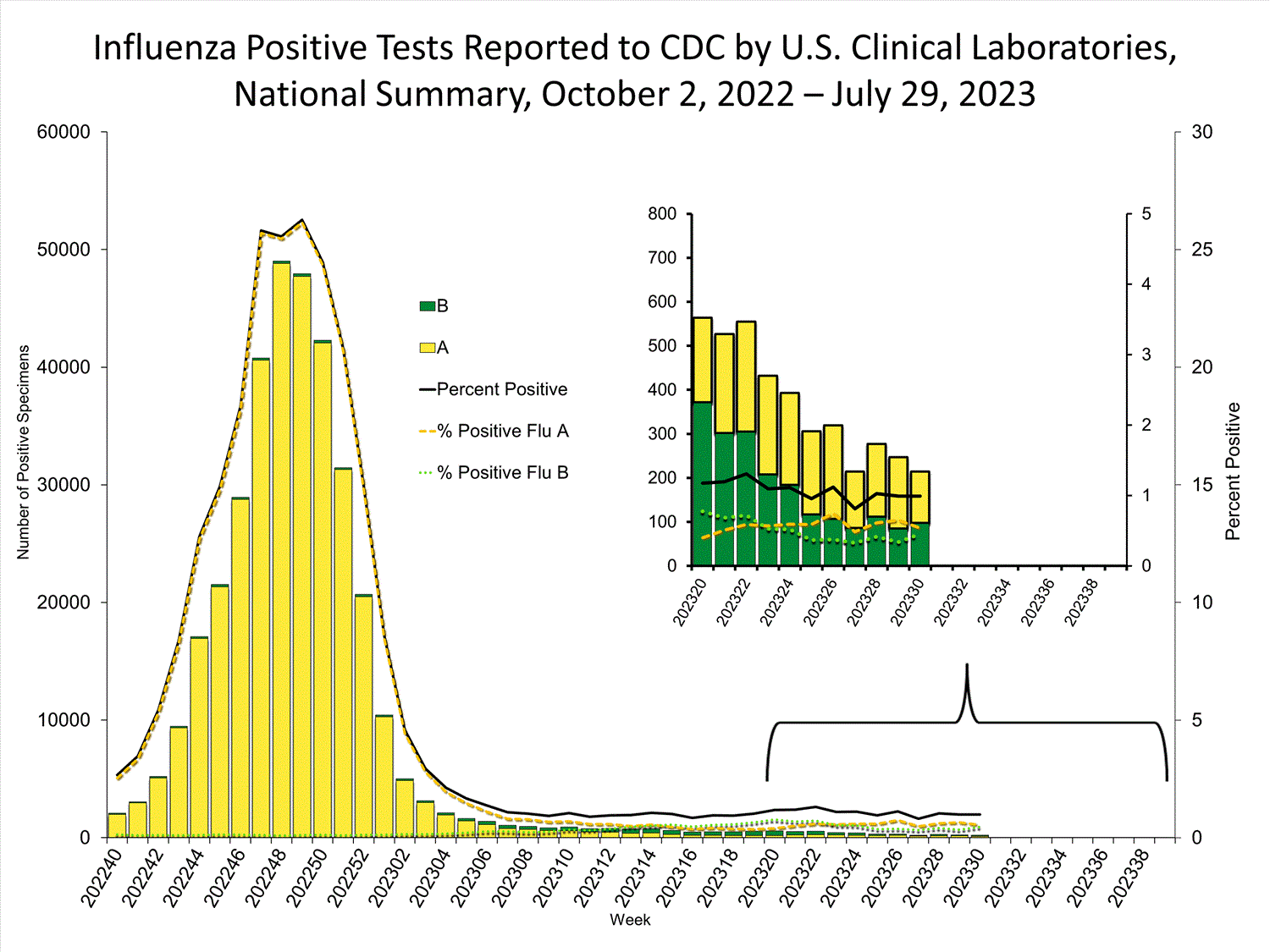
Clinical Laboratories
The results of tests performed by clinical laboratories nationwide are summarized below. Data from clinical laboratories (the percentage of specimens tested that are positive for influenza) are used to monitor whether influenza activity is increasing or decreasing.
| Week 30 | Data Cumulative since October 2, 2022(Week 40) |
|
|---|---|---|
| No. of specimens tested | 21,594 | 3,770,329 |
| No. of positive specimens (%) | 214 (1.0%) | 356,632 (9.5%) |
| Positive specimens by type | ||
| Influenza A | 116 (54.2%) | 347,674 (97.5%) |
| Influenza B | 98 (45.8%) | 8,958 (2.5%) |
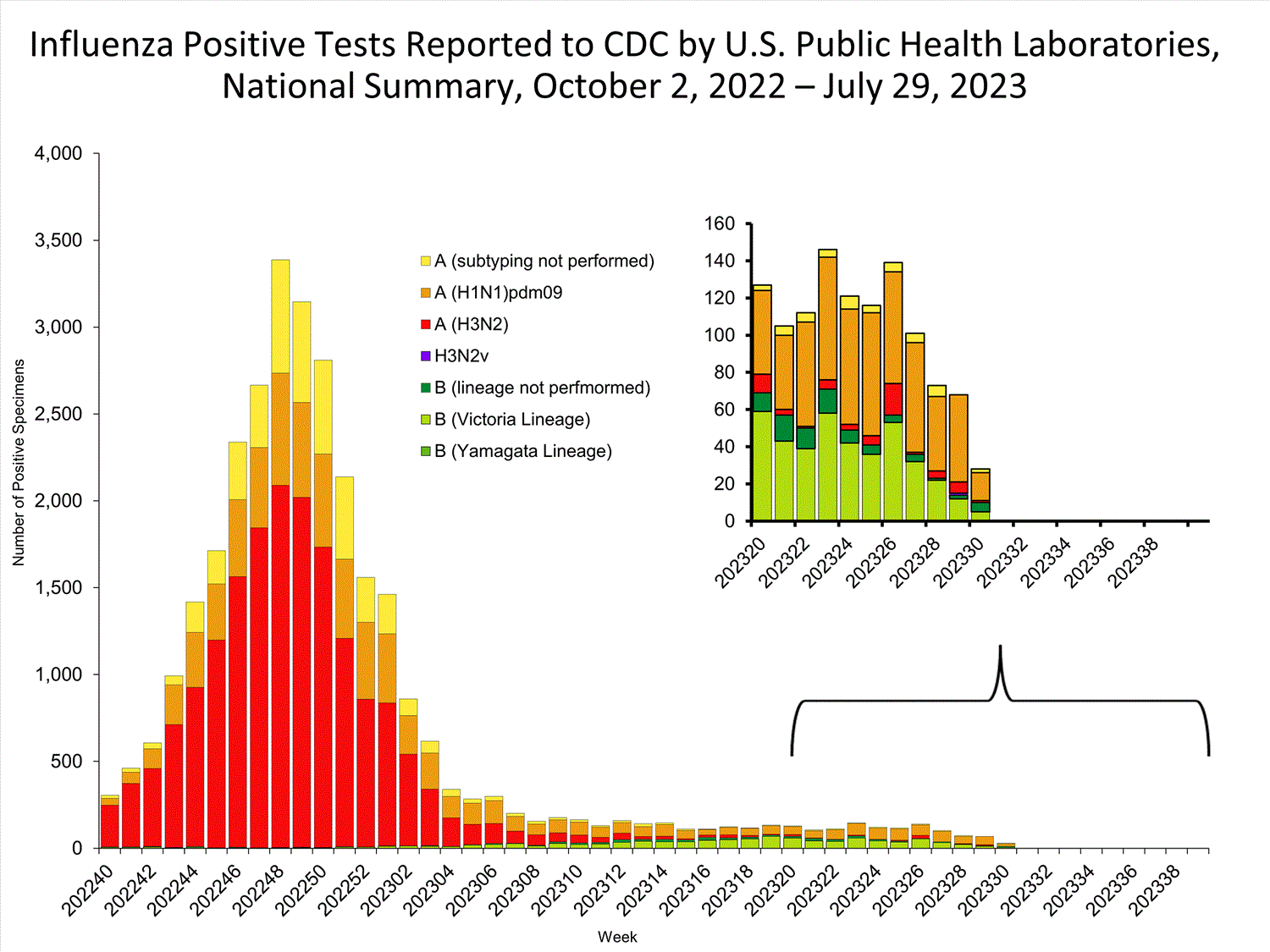
Public Health Laboratories
The results of tests performed by public health laboratories nationwide are summarized below. Data from public health laboratories are used to monitor the proportion of circulating viruses that belong to each influenza subtype/lineage. Viruses known to be associated with recent live attenuated influenza vaccine (LAIV) receipt or found upon further testing to be a vaccine virus are not included as they are not circulating influenza viruses.
| Week 30 | Data Cumulative since October 2, 2022 (Week 40) |
|
|---|---|---|
| No. of specimens tested | 1,261 | 271,803 |
| No. of positive specimens | 28 | 30,392 |
| Positive specimens by type/subtype | ||
| Influenza A | 18 (64.3%) | 29,120 (95.8%) |
| (H1N1)pdm09 | 15 (93.7%) | 7,128 (28.8%) |
| H3N2 | 1 (6.3%) | 17,648 (71.2%) |
| H3N2v | 0 | 2 (<0.1%) |
| Subtyping not performed | 2 | 4,342 |
| Influenza B | 10 (35.7%) | 1,272 (4.2%) |
| Yamagata lineage | 0 (0%) | 0 |
| Victoria lineage | 5 (100%) | 1,021 (100%) |
| Lineage not performed | 5 | 251 |
Additional virologic surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or Age Data
Novel Influenza A Virus
Two human infections with novel influenza A viruses were reported by the Michigan Department of Health and Human Services. One patient was infected with an influenza A(H3) variant (A(H3)v) virus, and the other patient was infected with an influenza A(H1N2)v virus.
The illness associated with influenza A(H3)v infection occurred during the week ending July 22, 2023 (week 29). An investigation by local public health officials found that prior to their illness onset the patient had swine exposure at an agricultural fair where influenza A virus was detected in swine.
The illness associated with influenza A(H1N2)v infection occurred during the week ending July 29, 2023 (week 30). An investigation by local public health officials found that prior to their illness onset the patient had swine exposure at an agricultural fair. This was not the same agricultural fair that the patient infected with influenza A(H3)v had attended.
Both patients are <18 years of age, were not hospitalized, received oseltamivir, and have recovered or are recovering from their illness. No person-to-person transmission of variant influenza A viruses associated with either patient has been identified. The investigations are ongoing.
These are the first variant influenza A viruses reported in the United States in 2023. When an influenza virus that normally circulates in swine (but not people) is detected in a person, it is called a “variant” influenza virus. Most human infections with variant influenza viruses occur following exposure to swine, but human-to-human transmission can occur. It is important to note that in most cases, variant influenza viruses have not shown the ability to spread easily and sustainably from person to person.
Early identification and investigation of human infections with novel influenza A viruses are critical so that the risk of infection can be understood, and appropriate public health measures can be taken.
Additional information on influenza in swine, variant influenza virus infection in humans, and guidance to interact safely with swine can be found at www.cdc.gov/flu/swineflu/index.htm.
Additional information regarding human infections with novel influenza A viruses:
Surveillance Methods | FluView Interactive: Novel Influenza
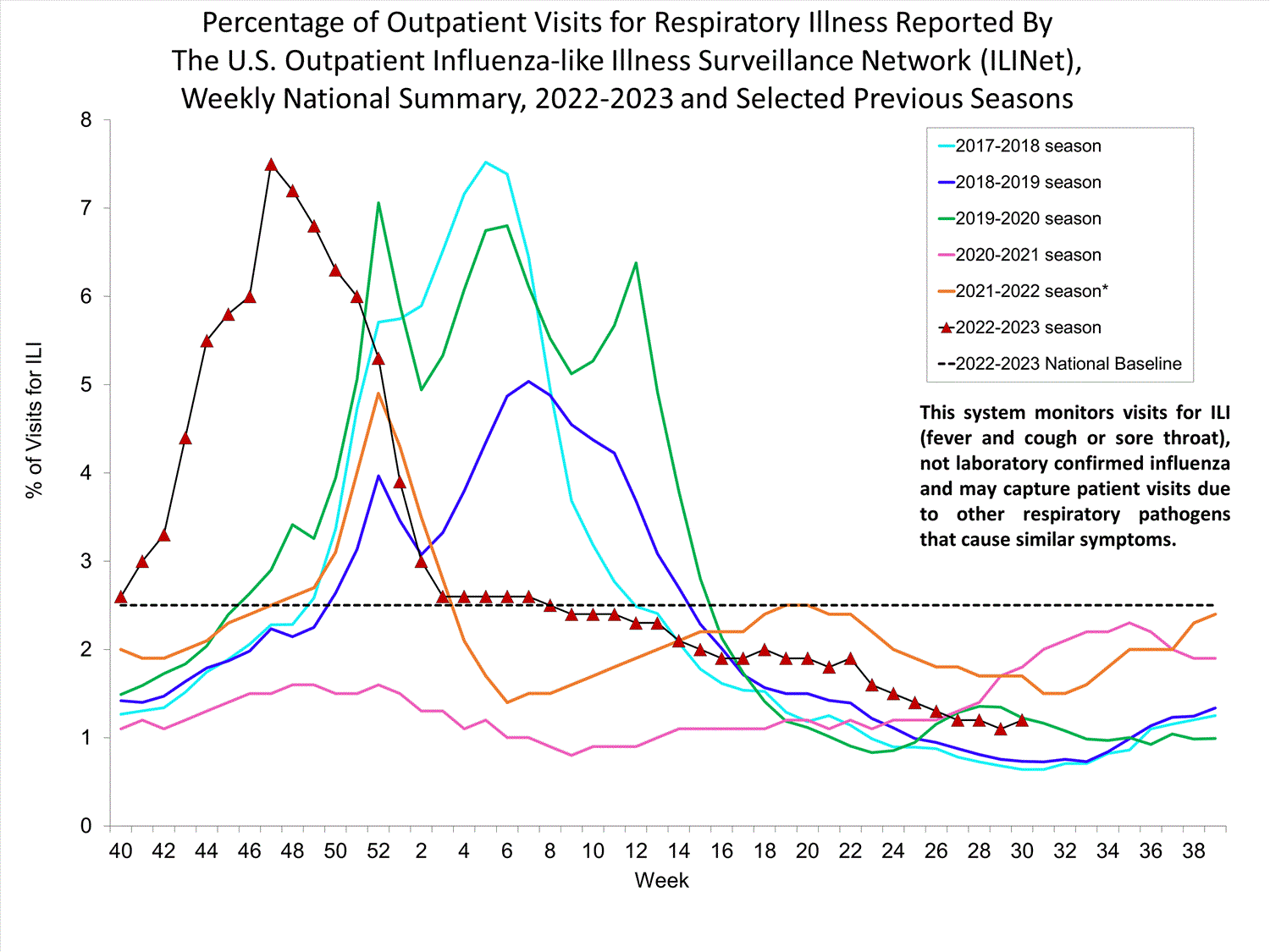
Outpatient Respiratory Illness Surveillance
The U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) monitors outpatient visits for respiratory illness referred to as influenza-like illness [ILI (fever plus cough or sore throat)], not laboratory-confirmed influenza, and may capture respiratory illness visits due to infection with any pathogen that can present with similar symptoms, including influenza, SARS-CoV-2, and RSV. Therefore, it is important to evaluate syndromic surveillance data, including that from ILINet, in the context of other sources of surveillance data to obtain a complete and accurate picture of influenza, SARS-CoV-2, and other respiratory virus activity. Other respiratory virus surveillance data can be found on CDC’s COVID Data Tracker, NCIRD Surveillance Systems website and National Respiratory and Enteric Virus Surveillance System (NREVSS) website.
Outpatient Respiratory Illness Visits
Nationwide during week 30, 1.2% of patient visits reported through ILINet were due to respiratory illness that included fever plus a cough or sore throat, also referred to as ILI. Multiple respiratory viruses are co-circulating, and the relative contribution of influenza virus infection to ILI varies by location.
* Effective October 3, 2021 (week 40), the ILI definition (fever plus cough or sore throat) no longer includes “without a known cause other than influenza.”
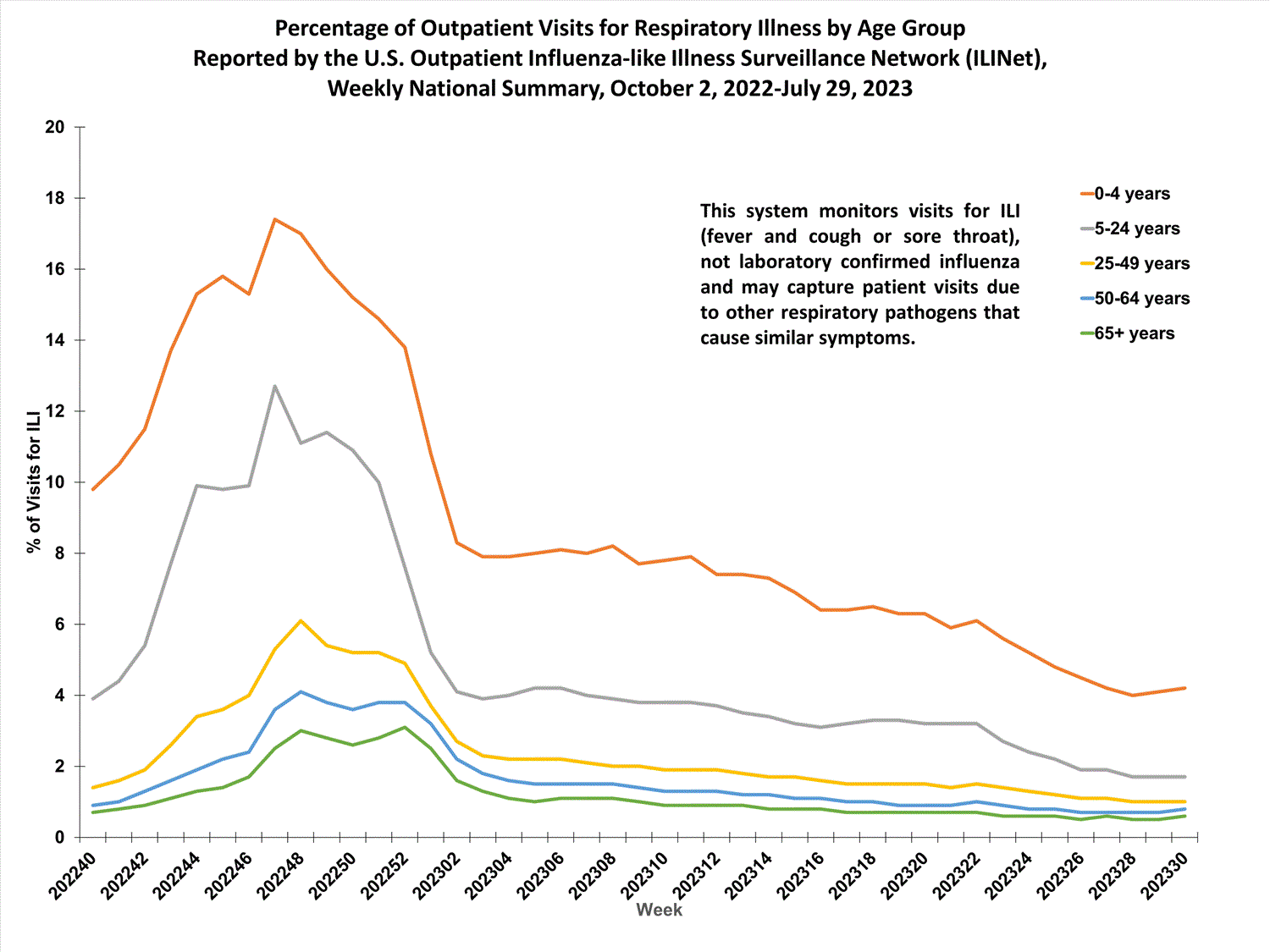
Outpatient Respiratory Illness Visits by Age Group
More than 70% of ILINet participants provide both the number of patient visits for respiratory illness and the total number of patient visits for the week broken out by age group. Data from this subset of providers are used to calculate the percentages of patient visits for respiratory illness by age group.
During week 30, the percentage of visits for respiratory illness reported in ILINet was 4.2% among those 0-4 years, 1.7% among those 5-24 years, 1.0% among those 25-49 years, 0.8% among those 50-64 years, and 0.6% among those 65 years and older.
Outpatient Respiratory Illness Activity Map
Data collected in ILINet are used to produce a measure of ILI activity* by state/jurisdiction and Core Based Statistical Areas (CBSA).
| Activity Level | Number of Jurisdictions | Number of CBSAs | ||
|---|---|---|---|---|
| Week 30
(Week ending |
Week 29
(Week ending |
Week 30
(Week ending |
Week 29
(Week ending |
|
| Very High | 0 | 0 | 0 | 0 |
| High | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 1 | 3 |
| Low | 0 | 0 | 15 | 9 |
| Minimal | 55 | 55 | 637 | 653 |
| Insufficient Data | 0 | 0 | 276 | 264 |
*Data collected in ILINet may disproportionally represent certain populations within a jurisdiction or CBSA, and therefore, may not accurately depict the full picture of influenza activity for the entire jurisdiction or CBSA. Differences in the data presented here by CDC and independently by some health departments likely represent differing levels of data completeness with data presented by the health department likely being the more complete.
Additional information about medically attended visits for ILI for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or ILI Activity Map
Hospitalization Surveillance
FluSurv-NET
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in select counties in 13 states and represents approximately 9% of the U.S. population. FluSurv-NET hospitalization data are preliminary. Patients admitted for laboratory-confirmed influenza-related hospitalization after April 30, 2023, will not be included in FluSurv-NET for the 2022-2023 season. Data on patients admitted through April 30, 2023, will continue to be updated on FluView Interactive as additional information is received.
Additional FluSurv-NET hospitalization surveillance information for current and past seasons and additional age groups:
Surveillance Methods |FluView Interactive: Rates by Age, Sex, and Race/Ethnicity or Data on Patient Characteristics | RESP-NET Interactive
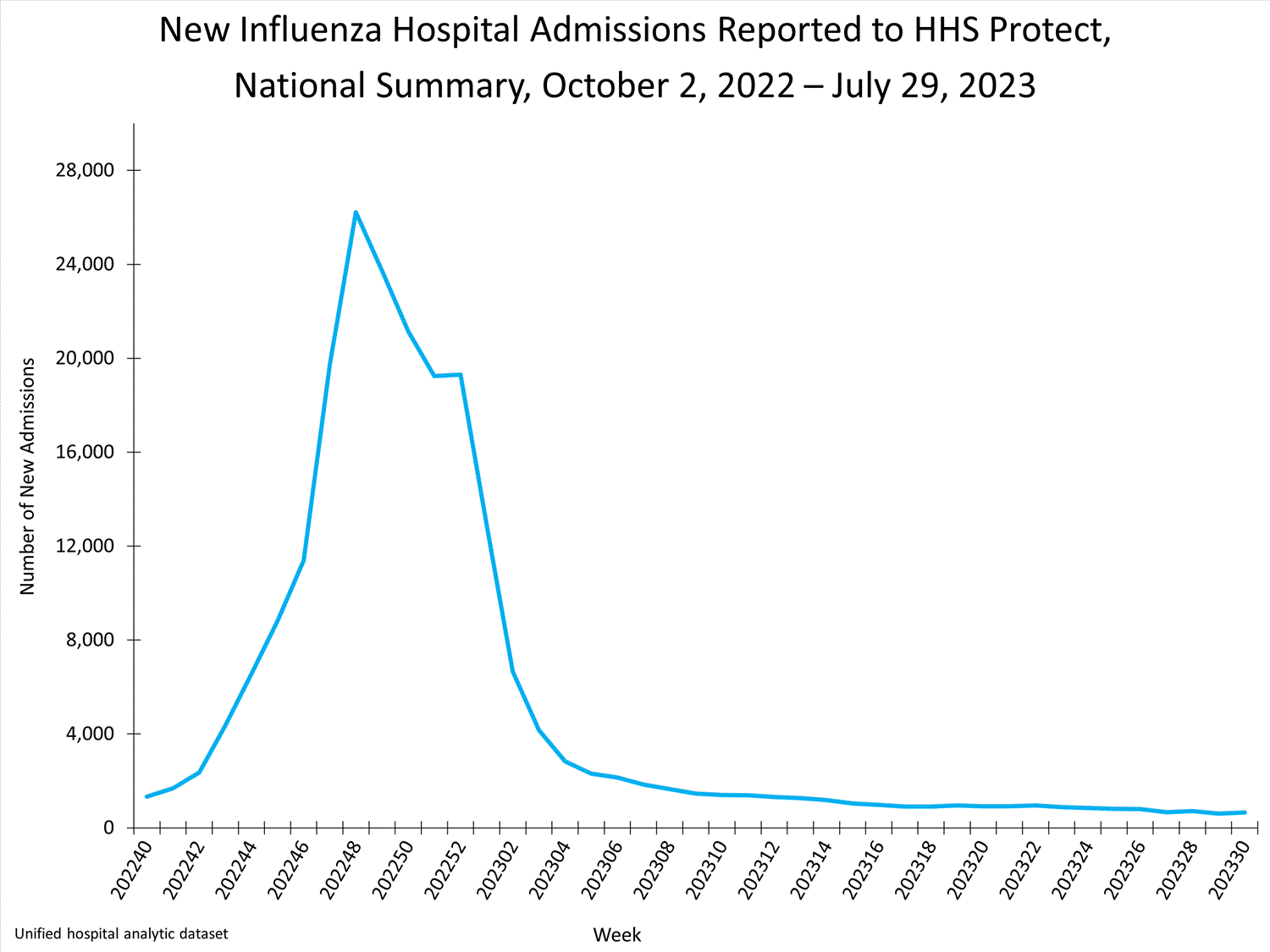
HHS Protect Hospitalization Surveillance
Hospitals report to HHS Protect the number of patients admitted with laboratory-confirmed influenza. During week 30, 649 patients with laboratory-confirmed influenza were admitted to a hospital.
Additional HHS Protect hospitalization surveillance information:
Surveillance Methods | Additional Data
Mortality Surveillance
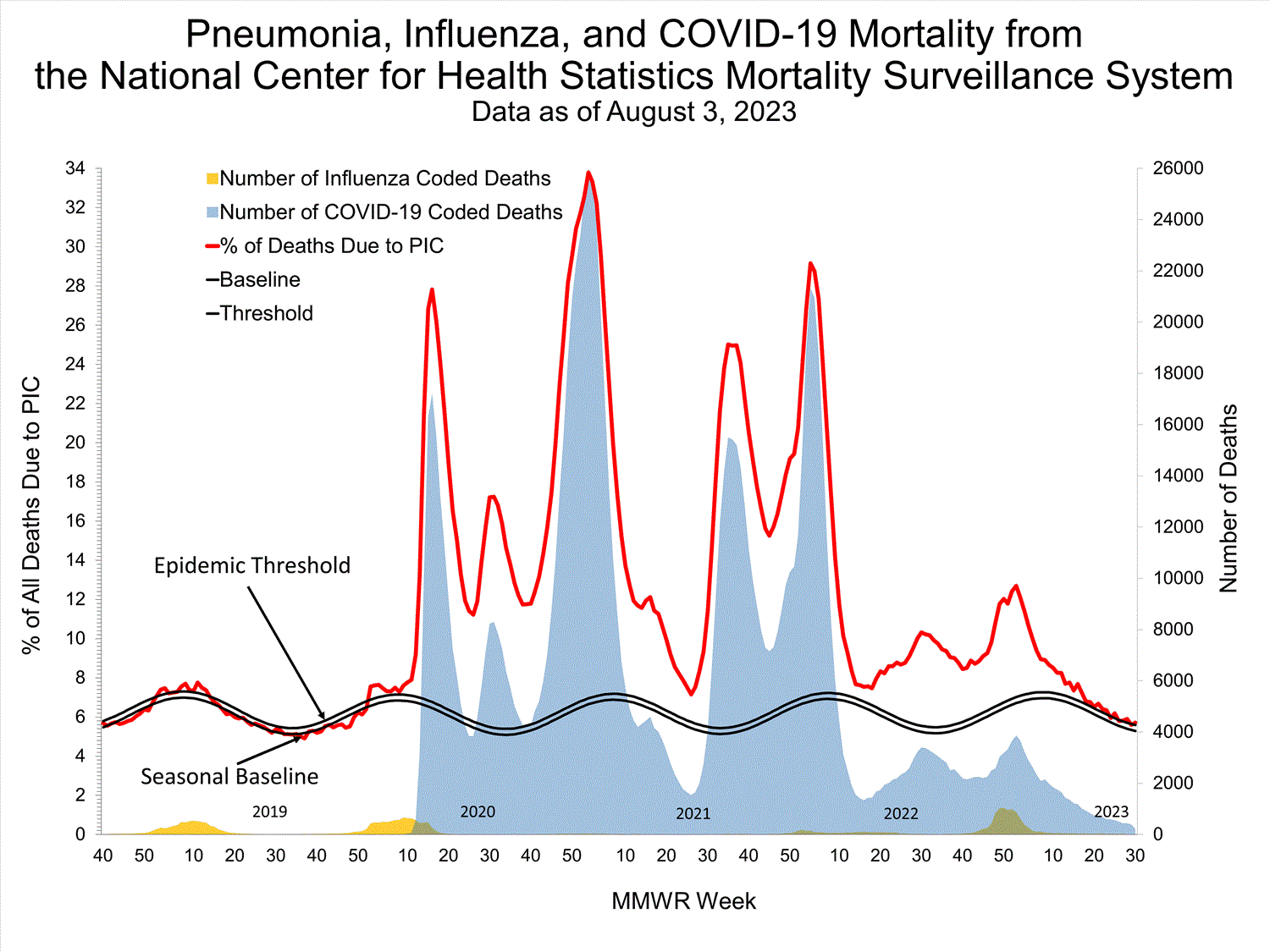
National Center for Health Statistics (NCHS) Mortality Surveillance
Based on NCHS mortality surveillance data available on August 3, 2023, 5.7% of the deaths that occurred during the week ending July 29, 2023 (week 30), were due to pneumonia, influenza, and/or COVID-19 (PIC). This percentage is above the epidemic threshold of 5.6% for this week. Among the 1,289 PIC deaths reported for this week, 218 had COVID-19 listed as an underlying or contributing cause of death on the death certificate, and four listed influenza. The data presented are preliminary and may change as more data are received and processed.
Additional pneumonia, influenza and COVID-19 mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
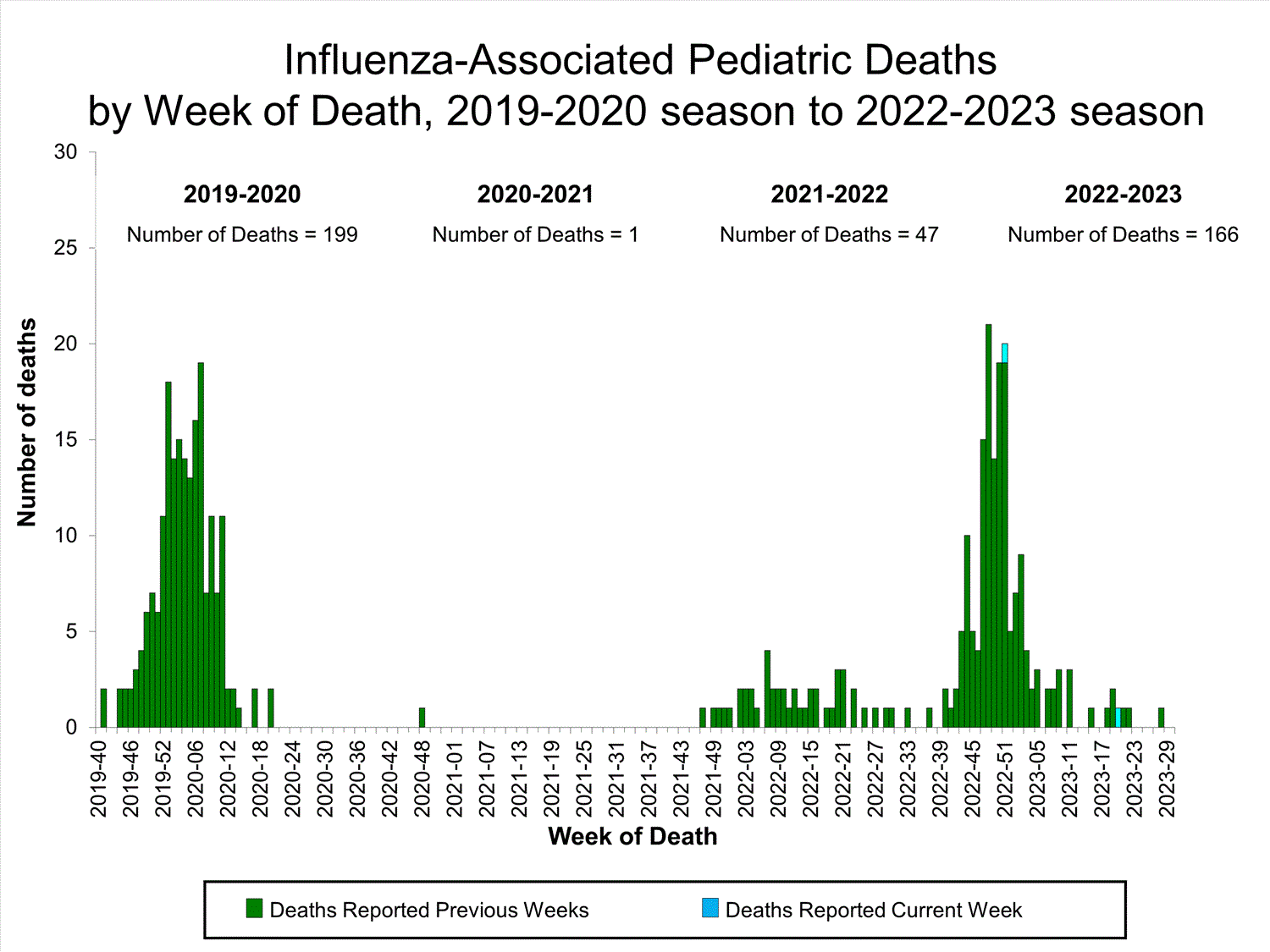
Influenza-Associated Pediatric Mortality
Two influenza-associated pediatric deaths occurring during the 2022-2023 season were reported to CDC during week 30. One death occurred during week 51 of 2022 (the week ending December 24, 2022) and one death occurred during week 20 of 2023 (the week ending May 20, 2023). Both deaths were associated with influenza A(H1N1) viruses.
A total of 166 influenza-associated pediatric deaths occurring during the 2022-2023 season have been reported to CDC.
Additional pediatric mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics.
National Institute for Occupational Safety and Health: Monthly surveillance data on the prevalence of health-related workplace absenteeism among full-time workers in the United States are available from NIOSH.
U.S. State and local influenza surveillance: Select a jurisdiction below to access the latest local influenza information.
World Health Organization:
Additional influenza surveillance information from participating WHO member nations is available through
FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza:
Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia)
Europe:
The most up-to-date influenza information from Europe is available from WHO/Europe and the European Centre for Disease Prevention and Control.
Public Health Agency of Canada:
The most up-to-date influenza information from Canada is available in Canada’s weekly FluWatch report.
Public Health England:
The most up-to-date influenza information from the United Kingdom is available from Public Health England.
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
A description of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component is available on the surveillance methods page.