Influenza Antiviral Drug Resistance
Questions & Answers
- What are reduced susceptibility and antiviral resistance?
- How widespread are reduced susceptibility and antiviral resistance in the United States?
- How does reduced susceptibility and antiviral resistance happen?
- How are reduced susceptibility and antiviral resistance detected?
- How has CDC prepared to test for reduced susceptibility and antiviral resistance to the new flu antiviral baloxavir?
- What is oseltamivir resistance and what causes it?
- How does CDC improve monitoring of influenza viruses for reduced susceptibility and antiviral resistance?
- How did influenza antiviral susceptibility patterns change during the previous (2021-2022) influenza season?
- What can people do to protect themselves against flu viruses with reduced susceptibility and antiviral resistance?
- What implications do reduced susceptibility and antiviral resistance have for the U.S. antiviral stockpile that was created as part of the United States pandemic plan?
What are reduced susceptibility and antiviral resistance?
When an antiviral drug is fully effective against a virus, that virus is said to be susceptible to that antiviral drug. Flu viruses are constantly changing, and some changes can make antiviral drugs work less well or not work at all against these viruses. Antiviral drugs work by targeting a specific location or site found on a flu virus. When a flu virus develops changes to the site that antiviral drugs target, that virus may show reduced or no susceptibility to that antiviral drug. Flu viruses can show reduced susceptibility to one or more flu antiviral drugs. Reduced susceptibility that is detected using laboratory methods can be a sign of potential antiviral drug resistance in clinical settings. Typically, a flu virus is called resistant after sufficient laboratory evidence is available to show that the antiviral drug lacks activity against the virus.
In the United States, there are four FDA-approved antiviral drugs recommended by CDC this season. Three are neuraminidase inhibitor antiviral drugs: oseltamivir (available as a generic version or under the trade name Tamiflu®) for oral administration, zanamivir (trade name Relenza®) for oral inhalation using an inhaler device, and peramivir (trade name Rapivab®) for intravenous administration. The fourth is a cap-dependent endonuclease inhibitor, baloxavir marboxil (trade name Xofluza®) for oral administration. Baloxavir marboxil was approved for use in the United States by FDA in October of 2018.
There is another class of FDA-approved antiviral drugs, M2 inhibitors amantadine and rimantadine, also called the adamantanes, that in the past were active against flu A viruses (but not flu B viruses). However, the adamantane antiviral drugs have not been recommended for use to treat flu in the United States for many years because of widespread antiviral resistance to this class of antivirals among circulating flu A viruses.
How widespread are reduced susceptibility and antiviral resistance in the United States?
In the United States, the majority of the recently circulating flu viruses have been fully susceptible to the neuraminidase inhibitors and to baloxavir. However, near all flu A viruses are resistant to the M2 inhibitors, which is why they are not recommended for treatment of seasonal influenza.
How does reduced susceptibility and antiviral resistance happen?
Flu viruses are constantly changing; they can change in significant ways from one season to the next and can even change within the course of one flu season. As a flu virus replicates (i.e., make copies of itself), its genetic makeup may change in a way that results in the virus becoming less susceptible to one or more of the antiviral drugs used to treat or prevent flu. Flu viruses can become less susceptible to antiviral drugs during the course of antiviral treatment or emerge spontaneously. Antiviral resistant flu viruses vary in their ability to infect people and are not necessarily more or less transmissible than susceptible flu viruses.
How are reduced susceptibility and antiviral resistance detected?
CDC routinely analyze flu viruses collected through domestic and global surveillance to see if they have genetic changes that are associated with reduced susceptibility to any flu antiviral drugs. Such changes can potentially cause viruses to be resistant to antiviral treatment with reduced or no effectiveness for treated patients. In addition, numerous state public health laboratories participate in screening of flu viruses for genetic changes indicative of potential resistance to neuraminidase inhibitor antivirals. CDC also is collaborating with the Wadsworth Center public health laboratory of New York State’s Department of Health (NYSDOH), a National Influenza Reference Center (NIRC), to establish additional laboratory-testing capacity for monitoring baloxavir antiviral susceptibility. This combined data informs public health policy recommendations about the use of flu antiviral medications.
CDC is continuously improving testing algorithms and methods used for monitoring antiviral susceptibility in circulating viruses. Detection of reduced susceptibility and antiviral resistance involves several laboratory tests, including phenotypic assays (testing in the presence of an antiviral drug) and molecular techniques (next generation sequence analysis and pyrosequencing) to look for genetic changes that have been associated with reduced antiviral susceptibility.
How has CDC prepared to test for reduced susceptibility and antiviral resistance to the new flu antiviral baloxavir?
CDC’s Influenza Division took specific laboratory actions to incorporate the antiviral drug baloxavir into routine virologic surveillance. This included the creation and validation of new assays to determine baloxavir susceptibility, and training of laboratorians to conduct baloxavir susceptibility testing.
Seasonal flu A and B viruses in humans as well as several flu A viruses that circulate in animals were tested to establish baseline susceptibility to baloxavir. In addition, the susceptibility of other distantly related flu viruses to baloxavir was tested. CDC also collaborated with the Association of Public Health Laboratories (APHL) and the Wadsworth Center NYSDOH, a National Influenza Reference Center (NIRC), to establish laboratory-testing capacity for baloxavir susceptibility. CDC has trained staff within these partner organizations to use CDC’s method for assessing baloxavir susceptibility.
What is oseltamivir resistance and what causes it?
Flu viruses are constantly changing (for more information, see How the Flu Virus Can Change.) Changes that occur in circulating flu viruses typically involve the structures of the viruses’ two primary surface proteins: hemagglutinin (HA) and neuraminidase (NA) (See image below for a visualization of a flu virus and its HA and NA surface proteins.)
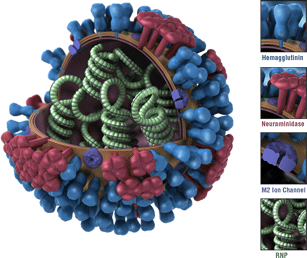
Oseltamivir is the most commonly prescribed antiviral drug of those recommended in the United States to treat flu illness. Oseltamivir is known as a “NA inhibitor” because this antiviral drug binds to NA proteins of a flu virus and inhibits the enzymatic activity of these proteins. By inhibiting NA activity, oseltamivir prevents flu viruses from spreading from infected cells to other healthy cells.
Changes in the NA proteins of a flu virus can reduce oseltamivir’s binding to them. As a result, oseltamivir’s ability to inhibit the enzyme activity of NA proteins can be diminished and this may cause “oseltamivir resistance” (non-susceptibility). A particular genetic change known as the “H275Y” mutation in the NA is the mutation that is known to confer oseltamivir resistance in A(H1N1)pdm09 flu viruses. Flu viruses that have the “H275Y” mutation show highly reduced inhibition by oseltamivir in laboratory assays. The “H275Y’ mutation makes oseltamivir ineffective in treating illnesses with that flu virus by preventing oseltamivir from inhibiting NA activity, which then allows the virus to infect healthy cells. The “H275Y” mutation also reduces the effectiveness of peramivir to treat infections caused by a flu virus with this mutation. Some other mutations in the NA proteins of circulating viruses have been shown to affect oseltamivir’s ability to inhibit the enzyme activity of the viruses’ NA proteins. Such viruses can show ‘reduced’ or even ‘highly reduced’ inhibition by oseltamivir and other NA inhibitors in laboratory tests; however, not all are considered “resistant” due to insufficient data to support their drug resistance from clinical settings.
How does CDC improve monitoring of influenza viruses for reduced susceptibility and antiviral resistance?
CDC continually improves the ability to rapidly detect flu viruses with antiviral reduced susceptibility and antiviral resistance through improvements in laboratory methods; increasing the number of surveillance sites in the U.S. and worldwide; and increasing the number of laboratories that can test for reduced susceptibility and antiviral resistance. Enhanced surveillance efforts have provided CDC with the capability to detect antiviral resistant flu viruses more quickly, and enabled CDC to monitor for changing trends over time.
How did influenza antiviral susceptibility patterns change during the previous (2021-2022) influenza season?
Antiviral susceptibility patterns changed very little in 2021-2022 compared with the previous season (2020-2021). During the 2021-2022 and 2020-2021 seasons, only a very small of viruses were found to be resistant to oseltamivir. Almost all of the flu viruses tested during 2021-2022 continued to be susceptible to the antiviral drugs recommended for treatment of flu by the Centers for Disease Control and Prevention (CDC). Resistance to the adamantane class of antiviral drugs among flu A(H3N2) and A(H1N1) pdm09 viruses remained widespread (flu B viruses are not susceptible to adamantane drugs).
CDC conducts ongoing surveillance and testing of flu viruses for antiviral reduced susceptibility and resistance among seasonal and novel flu A viruses (of animal origin that have infected people), and guidance is updated as needed.
Because there were no dramatic changes in antiviral susceptibility patterns during the 2021-2022 flu season, the guidance on the use of flu antiviral drugs for the 2022-2023 flu season remains unchanged. The latest guidance for clinicians on the use of antiviral drugs for flu is available on the CDC web site at Antiviral Drugs: Information for Health Professionals.
What can people do to protect themselves against flu viruses with reduced susceptibility and antiviral resistance?
Getting a yearly seasonal flu vaccination is the best way to reduce the risk of flu and its potentially serious complications. Flu vaccines protect against influenza A viruses of two subtypes, A(H1N1)pdm09 and A(H3N2), and type B viruses from two lineages. CDC recommends that everyone 6 months of age and older get vaccinated each year. If you are in a group at higher risk of serious flu-related complications and become ill with flu symptoms, call your doctor right away, because you might benefit from early treatment with a flu antiviral drug. If you are not at higher risk of flu complications, if possible, stay home from work, school and errands when you are sick. This will help prevent you from spreading your illness to others. See Important Information For People Sick With Flu for more information.
What implications do reduced susceptibility and antiviral resistance have for the U.S. antiviral stockpile that was created as part of the United States pandemic plan?
Monitoring for antiviral drug susceptibility and resistance will be essential to determine the role of antivirals and specific antivirals during the next influenza pandemic. Available FDA-approved and authorized flu antiviral drugs can be used in the event that a novel flu A virus, such as avian flu A(H7N9) virus, gains the ability to spread easily among people in a sustained manner, and is susceptible to these antiviral drugs. During the 2009 H1N1 pandemic, neuraminidase inhibitor antiviral drugs were released from the Strategic National Stockpile (SNS) and used to treat infection with the pandemic virus, now referred to as flu A(H1N1) pdm09 virus. In addition, an investigational antiviral drug was made available by FDA Emergency Use Authorization for treatment of hospitalized pandemic influenza patients in the U.S. through clinician requests to CDC. Antivirals in the SNS are for use during public health emergencies in the United States, such as a flu pandemic, but not for seasonal flu epidemics. CDC no longer oversees the SNS. More information is available at Strategic National Stockpile.
