Malaria Diagnostic Tests
Microscopic examination remains the “gold standard” for laboratory confirmation of malaria. These tests should be performed immediately when ordered by a health-care provider. They should not be saved for the most qualified staff to perform or batched for convenience. In addition, these tests should not be sent out to reference laboratories with results available only days to weeks later. It is vital that health-care providers receive results from these tests within hours in order to appropriately treat their patients infected with malaria.
Technique
(See DPDx specimen preparation) A blood specimen collected from the patient is spread as a thick or thin blood smear, stained with a Romanovsky stain (most often Giemsa), and examined with a 100X oil immersion objective. Visual criteria are used to detect malaria parasites and to differentiate (when possible) the various species. (See DPDx Plasmodium species comparison chart) Wright’s stain, which is commonly used in hospital laboratories for examining blood (called a CBC with manual differential), can be used if Giemsa stain is not available. However, species determination might be more difficult.
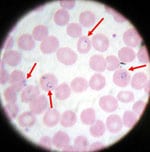
Blood smear from a patient with malaria; microscopic examination shows Plasmodium falciparum parasites (arrows) infecting some of the patient’s red blood cells. (CDC photo)
Advantages
Microscopy is an established, relatively simple technique that is familiar to most laboratory scientists. Any laboratory that can perform routine hematology tests is equipped to perform a thin and thick malaria smear. Within a few hours of collecting the blood, the microscopy test can provide valuable information. First and foremost it can determine that malaria parasites are present in the patient’s blood. Once the diagnosis is established – usually by detecting parasites in the thick smear – the laboratory scientist can examine the thin smear to determine the malaria species and the parasitemia, or the percentage of the patient’s red blood cells that are infected with malaria parasites. The thin and thick smears are able to provide all 3 of these vital pieces of information to the doctor to guide the initial treatment decisions that need to be made acutely.
Disadvantages
Microscopy results are only as reliable as the laboratories performing the tests. In the United States, there are, on average, 2000 cases of malaria diagnosed and reported each year. Thus, the average laboratory scientist does not perform this test regularly, and may not be maintaining optimal proficiency.
A Rapid Diagnostic Test (RDT) is an alternate way of quickly establishing the diagnosis of malaria infection by detecting specific malaria antigens in a person’s blood. RDTs have recently become available in the United States.
Technique
A blood specimen collected from the patient is applied to the sample pad on the test card along with certain reagents. After 15 minutes, the presence of specific bands in the test card window indicate whether the patient is infected with Plasmodium falciparum or one of the other 3 species of human malaria. It is recommended that the laboratory maintain a supply of blood containing P. falciparum for use as a positive control.
Advantages
High-quality malaria microscopy is not always immediately available in every clinical setting where patients might seek medical attention. Although this practice is discouraged, many healthcare settings either save blood samples for malaria microscopy until a qualified person is available to perform the test, or send the blood samples to commercial or reference laboratories. These practices have resulted in long delays in diagnosis. The laboratories associated with these health-care settings may now use an RDT to more rapidly determine if their patients are infected with malaria.

BinaxNOW® Malaria Test, the only available RDT for malaria in the United States.
Disadvantages
The use of the RDT does not eliminate the need for malaria microscopy. The RDT may not be able to detect some infections with lower numbers of malaria parasites circulating in the patient’s bloodstream. Also, there is insufficient data available to determine the ability of this test to detect the 2 less common species of malaria, P. ovale and P. malariae. Therefore all negative RDTs must be followed by microscopy to confirm the result.
In addition, all positive RDTs also should be followed by microscopy. The currently approved RDT detects 2 different malaria antigens; one is specific for P. falciparum and the other is found in all 4 human species of malaria. Thus, microscopy is needed to determine the species of malaria that was detected by the RDT. In addition, microscopy is needed to quantify the proportion of red blood cells that are infected, which is an important prognostic indicator.
Training
On June 13, 2007, the FDA approved the first rapid diagnostic test (RDT) for use in the United States, the Binax NOW Malaria. This RDT is approved for use by hospital/commercial laboratories, not by individual clinicians or by patients themselves. This video provides guidance regarding the test procedure and results interpretation.
Indirect Fluorescent Antibody Test
Malaria antibody detection is performed using the indirect fluorescent antibody (IFA) test. The IFA procedure can be used to determine if a patient has been infected with Plasmodium. Because of the time required for development of antibody and also the persistence of antibodies, serologic testing is not practical for routine diagnosis of acute malaria. However, antibody detection may be useful for:
- Screening blood donors involved in cases of transfusion-induced malaria when the donor’s parasitemia may be below the detectable level of blood film examination
- Testing a patient, usually from an endemic area, who has had repeated or chronic malaria infections for a condition known as tropical splenomegaly syndrome
- Testing a patient who has been recently treated for malaria but in whom the diagnosis is questioned.
Species-specific testing is available for three of the four human species: P. falciparum, P. vivax, and P. malariae. P. ovale antigens are not always readily available and so antibody testing is not performed routinely. Cross reactions often occur between Plasmodium species and Babesia species. Blood stage Plasmodium species schizonts (meronts) are used as antigen. The patient’s serum is exposed to the organisms; homologous antibody, if present, attaches to the antigen, forming an antigen-antibody (Ag-Ab) complex. Fluorescein-labeled anti-human antibody is then added, which attaches to the patient’s malaria-specific antibodies. When the slide is examined with a fluorescence microscope, if parasites fluoresce an apple green color, a positive reaction has occurred.
Enzyme immunoassays have also been employed as a tool to screen blood donors, but have limited sensitivity due to use of only Plasmodium falciparum antigen instead of antigens of all four human species.
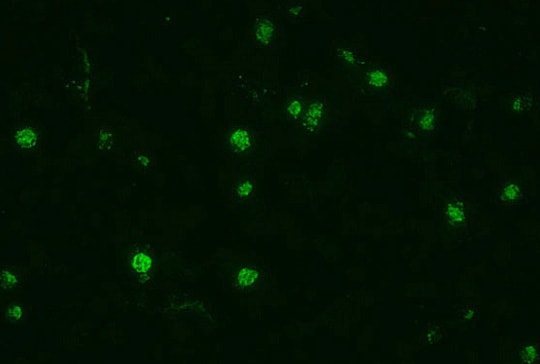
Indirect fluorescent antibody (IFA) test. The fluorescence indicates that the patient serum being tested contains antibodies that are reacting with the antigen preparation (here, Plasmodium falciparum parasites).
