Asthma control
- Components of asthma control
- Classification of asthma control for each component
- Overall classification of asthma control across 4 components
- Categorizing asthma symptoms (Control_1)
- Categorizing nighttime awakenings (Control_2)
- Categorizing interference with activity (Control_3):
- Categorizing short-activing beta agonists use (Control_4)
Asthma control is based on the most impaired level across four components or variables. Control classification is dichotomized into “well controlled” versus “not well controlled/very poorly controlled.” This is a simplification of the NAEPP Guideline (EPR-3) control categories where “not well controlled” and “very poorly controlled” are separate categories.
Symptoms: “During the past 30 days, on how many days did [child’s name] have any symptoms of asthma? (SYMP_30D)”
Nighttime awakenings:
”During the past 30 days, on how many days did symptoms of asthma make it difficult for [him/her] to stay asleep? (ASLEEP30)”
Interference with normal activity: “During just the past 30 days, would you say [child’s name] limited [his/her] usual activities due to asthma not at all, a little, a moderate amount, or a lot? (ACT_DAYS30 )” Note: the recall period for this question changed in the ACBS from “12 months” to “30 days” in 2012.
Short-acting beta agonist use: Requires an algorithm using responses from 4 questions:
“In the past 3 months has [child’s name] taken prescription asthma medicine using an inhaler? (INH_SCR)“
“How many times per day or per week does [he/she] use [MEDICATION FROM INH_MEDS SERIES]1? (ILP08_#)”
“In the past 3 months, did [he/she] take [MEDICATION FROM INH_MEDS SERIES]1 when [he/she] had an asthma episode or attack? (ILP04_#)”
“In the past 3 months, did [he/she] take [MEDICATION FROM INH_MEDS SERIES]1 before exercising? (ILP05_#)”
1INH_MEDS series is a list of medications which the interviewer uses to identify up to 8 medications reported by the respondent. This list should be checked in the ACBS documentation each year for updates. In 2012, it included the following SABA medications: albuterol (Ventolin, Proair HFA, Proventil), bitolterol (Tornalate), levalbuterol (Xopenex), metaproterenol (Alupent), pirbuterol (Maxair), salbutamol (albuterol), and terbutaline (Brethaire).
Classification of asthma control for each component:

* Response code “88” corresponds to zero or no days.
Overall classification of asthma control across 4 components:
Code control for each of the indices below (Symptoms, Nighttime Awakenings, and SABA Use) into 4 separate variables. Then create a control variable based on the most impaired level across the 4.
Table 1
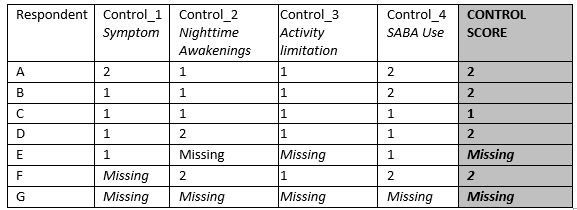
1= Well Controlled
2= Not Well/Very Poorly Controlled
Assign missing value for CONTROL_SCORE when 2 or more components have missing data (scenarios E and G in Table 1)
Categorizing asthma symptoms (Control_1)
BRFS Call Back item used: SYMP_30D
SYMP_30D question wording: “During the past 30 days, on how many days did [child’s name] have any symptoms of asthma?”
Level of control for this index is determined the same way for all 3 age pediatric groups. They are left stratified in Table 2 to demonstrate differences in the EPR-3 Guidelines by age group.
Table 2
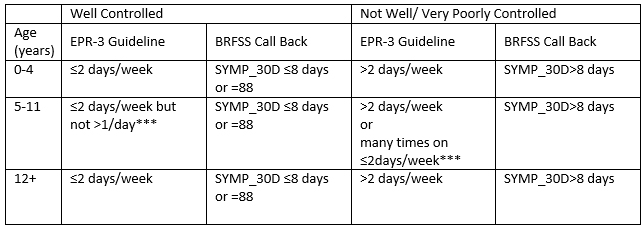
*** Cannot be measured since we don’t have the number of times per day asthma symptoms were experienced
Exclude responses SYMP_30D=77 or 99 (don’t know or refused to answer)
CUT POINT DETERMINATION
SYMP_30D: measures # days the respondent had symptoms in the past 30 days. The following demonstrates how the cut points in the table above were derived for all age groups above:
Table 3

Categorizing nighttime awakenings (Control_2):
BRFS Call Back item used: ASLEEP30
ASPEEP30 question wording: ”During the past 30 days, on how many days did symptoms of asthma make it difficult for [him/her] to stay asleep?”
Table 7
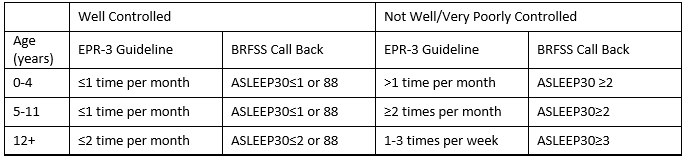
Note: 88=code for no days
Exclude responses ASLEEP30=77 or 99 (don’t know or refused to answer)
CUT POINT DETERMINATION
ASLEEP30: measures # days the respondent had difficulty staying asleep in the past 30 days due to asthma symptoms. The following demonstrates how the cut points in the table above were derived:
Table 8
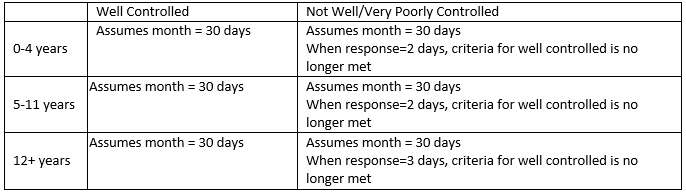
Categorizing interference with activity (Control_3):
BRFS Call Back item used: ACT_DAYS
ACT_DAYS question wording: “During just the past 30 days, would you say [child’s name] limited [his/her] usual activities due to asthma not at all, a little, a moderate amount, or a lot?”
Note: the recall period for this question changed in the ACBS from “12 months” to “30 days” in 2012.
For this component of asthma control, the EPR-3 Guidelines are the same for all pediatric age groups:

Exclude responses with ACT_DAYS = 7 or 9 (don’t know or refused to answer)
Categorizing short-activing beta agonists use (Control_4):
- BRFS Call Back items used: INH_SCR, INH_MEDS, ILP08, ILP04, ILP05
- INH_SCR question wording: “In the past 3 months has [child’s name] taken prescription asthma medicine using an inhaler?“
- INH_MEDS question wording: “How many times per day or per week does [he/she] use?”
- ILP04 question wording: “In the past 3 months, did [he/she] take [MEDICINE FROM INH_MEDS SERIES] when [he/she] had an asthma episode or attack?”
- ILP05 question wording: “In the past 3 months, did [he/she] take [MEDICINE FROM INH_MEDS SERIES] before exercising?”
- ILP08 question wording: “How many times per day or per week did [he/she] use [MEDICINE FROM INH_MEDS SERIES]?
- Level of control for this index is determined the same way for all 3 age groups, using inhaled medications only. The frequency of using nebulized medications are not available from the survey and therefore cannot be assessed for asthma control.
Process:
- Identify all those respondents who have taken a prescription asthma medicine using an inhaler (INH_SCR: 1)
- Identify those medications that are inhaled SABA (INH_MEDS: 3, 4, 9, 10, 20, 21, 23, 24, 28, 30, 33, 37, 38, 41. These are SABA inhalers.). This determination needs annual update because new inhaled medications may be added to the survey. This list should be checked in the ACBS documentation each year for updates. In 2012, it included the following SABA medications: albuterol (Ventolin, Proair HFA, Proventil), bitolterol (Tornalate), Combivent, levalbuterol (Xopenex), metaproterenol (Alupent), pirbuterol (Maxair), salbutamol (albuterol), and terbutaline (Brethaire). Limitation: This indicator ONLY includes SABA medications taken by inhaler since the frequency of SABAs taken in some other form (including nebulizer) is not captured on the survey. Therefore, control assessed by SABA use will be an underestimate and may pose a severe limitation, especially to the 0-4 year olds.
- Determine which SABA medications where there is evidence that they were taken in the past 3 months only for treatment before exercise. For responses consistent with SABA use only before exercise, exclude from SABA total use according to Table 9.
- Then determine the frequency of use (ILP08) for the remaining responses and convert all to # times per day.
- Code Control_4 using Table 11.
Notes:
- If ILP08=555 (Never), contribute “0” per day to the total.
- IF ILP08=666 (less often than 1 per week), contribute as 1 per 10 day, or 0.10 per day.
- When data is missing or the response is Don’t Know or Refused for ILP08 (frequency use of INHALER medications), set the contribution of that SABA to the total to “0” so as to prevent missing values for the total SABA meds variable. If all reported SABAs have ILP08 = Don’t Know, Refused, or missing, then set the Total SABA Med Frequency to “missing” See Table 10, response scenarios M, N, O and P.
- If none of their reported asthma medications in the past 3 months are SABAs, then their total SABA frequency per day is “0” and they are “well controlled” for CONTROL_4.
- See Table 10 for example response scenarios.
Table 9
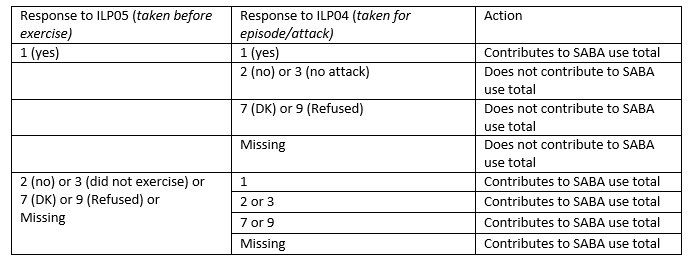
Table 10. Some Examples of Possible Response Scenarios
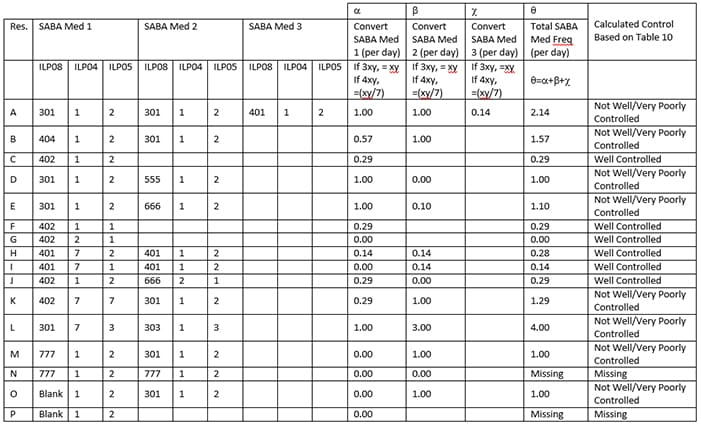
Table 11
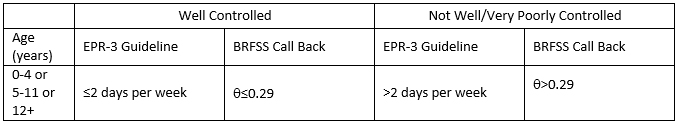
CUT POINT DETERMINATION
Table 12
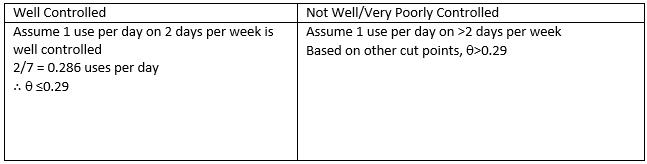
Note: 555 = Never…score as 0.00 per day
666 = less often than 1 per week…score as 1 per 10 days…or 0.10 per day
Where INH_SCR: 2 or where none of the inhaled meds are SABAs, code to well controlled