CDC’s Dye Test Detects Counterfeit Antimalarials
A colorimetric test developed at CDC to assess the quality of artesunate requires only a small portion of an artesunate tablet (1%), enabling the tablet to remain available for treatment. After the material is exposed to a strong base and then treated with a reagent, a distinct yellow color is produced if artesunate is present.
Colorimetry uses chemical reactions to transform the active ingredient into a colored compound. This method is convenient because it is very rapid, highly specific and inexpensive. The intensity of a positive color reaction is proportional to drug concentration, so visual assessment can easily be made for semiquantitative purposes by comparison with standards of known concentration. Color intensity can be measured using a portable filter photometer to give a quantitative assessment.
This test has been used to assess the quality of artesunate in Southeast Asia and Africa. It is routinely used by some nongovernmental organizations and hospitals to screen artesunate tablets for authenticity. Drug quality studies using the colorimetric test have found 38-53% of artesunate tablets from Cambodia, Laos, Myanmar (Burma), Thailand, and Vietnam did not contain the active ingredient. In a recent study in Laos, 22 of 25 (88%) outlets sold counterfeit artesunate. Cost and physical appearance of the tablets and packaging helped predict whether or not the drugs were authentic.
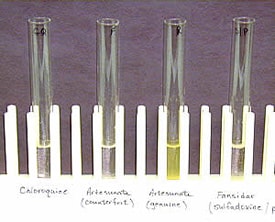
Colorimetric test for artesunate. From left to right: chloroquine, counterfeit artesunate, genuine artesunate, sulfadoxine-pyrimethamine. The genuine artesunate is distinguished by a positive yellow appearance.