Viral Hepatitis Surveillance Report 2019 – Introduction
BACKGROUND
Hepatitis A is a vaccine-preventable liver disease caused by the hepatitis A virus (HAV). HAV is usually transmitted person-to-person through the fecal–oral route or through consumption of contaminated food or water. The majority of adults and older children with hepatitis A have symptoms that usually resolve ≤2 months after infection; children aged <6 years usually do not have symptoms, or they have an unrecognized infection. Signs and symptoms associated with hepatitis A can include ≥1 of the following: fever, fatigue, nausea, vomiting, loss of appetite, abdominal pain, dark urine, and clay-colored stools. Hepatitis A is a self-limited disease that does not result in chronic infection. Treatment for HAV infection might include rest, adequate nutrition, and fluids. Hospitalization might be required for more severe cases. The best way to prevent hepatitis A is by being vaccinated (1).
Hepatitis B is a vaccine-preventable liver disease caused by the hepatitis B virus (HBV). HBV is transmitted when blood, semen, or another body fluid from a person infected with the virus enters the body of someone who is uninfected. This can happen through sexual contact; sharing needles, syringes, or other drug-injection equipment; or from mother to baby at birth. For some persons, hepatitis B is an acute, or short-term, illness; for others, it can become a long-term, chronic infection. Chronic hepatitis B can lead to serious health problems, including cirrhosis, liver cancer, and death. Treatments are available, but no cure exists for hepatitis B. The best way to prevent hepatitis B is by being vaccinated (2,3).
Hepatitis C is a liver disease caused by the hepatitis C virus (HCV). HCV is a bloodborne virus. Today in the United States, the majority of persons become infected with HCV by sharing needles or other equipment used in injecting drugs (4). For certain persons, hepatitis C is a short-term illness, but for >50% of persons who become infected with the HCV, it becomes a long-term, chronic infection (5). Like chronic hepatitis B, chronic hepatitis C is a serious disease that can result in cirrhosis, liver cancer, and death. Persons might not be aware of their infection because they are not clinically ill. However, since 2013, a highly effective, well-tolerated curative treatment has been available for hepatitis C, but no vaccine for preventing hepatitis C is yet available (6). The best way to prevent hepatitis C is by avoiding behaviors that can spread the disease, especially injecting drugs.
Key facts about hepatitis A, hepatitis B, and hepatitis C
| Characteristic | Hepatitis A | Hepatitis B | Hepatitis C |
|---|---|---|---|
| Main route(s) of transmission | Fecal-oral | Blood, sexual | Blood |
| Incubation period | 15–50 days (average: 28 days) |
60–150 days (average: 90 days) |
14–182 days (average range: 14–84 days) |
| Symptoms of acute infection | Symptoms are similar and can include ≥1 of the following: jaundice, fever, fatigue, loss of appetite, nausea, vomiting, abdominal pain, joint pain, dark urine, clay-colored stool, diarrhea (hepatitis A only) | ||
| Perinatal transmission | No | Yes | Yes |
| Vaccine available | Yes | Yes | No |
| Treatment | Supportive care | Yes, not curative | Yes, curative |
NATIONAL PROFILE OF VIRAL HEPATITIS, 2019
The Centers for Disease Control and Prevention (CDC) collects, analyzes, and disseminates viral hepatitis surveillance data. Each week, staff at health departments submit case reports of viral hepatitis to CDC through the National Notifiable Diseases Surveillance System (NNDSS). The annual surveillance report, published by the CDC, summarizes information about reported cases of hepatitis A, hepatitis B, and hepatitis C and deaths with any of these hepatitides listed as a cause of death in CDC’s National Vital Statistics System (NVSS). These surveillance data are used by public health partners to help focus prevention efforts, plan services, allocate resources, develop policy, and detect and respond to clusters of viral hepatitis infection. These actions support the goal of CDC’s Division of Viral Hepatitis 2020 – 2025 Strategic Plan (7) for establishing comprehensive national viral hepatitis surveillance for public health action.
The 2019 Viral Hepatitis Surveillance Report contains 21 tables and 25 figures, and there are some notable additions to the 2018 Viral Hepatitis Surveillance Report (8). For the first time, the Surveillance Report describes demographic characteristics of persons with chronic hepatitis B and chronic hepatitis C by age group, sex, race/ethnicity, and US Department of Health and Human Services regions. Additionally, the number and rates of viral hepatitis cases by urbanicity status is included for hepatitis A, acute and chronic hepatitis B, and acute and chronic hepatitis C infections. Finally, outcome data from CDC’s Perinatal Hepatitis B Prevention Program for infants born during 2018 to persons with HBV infection are reported from 64 jurisdictions.
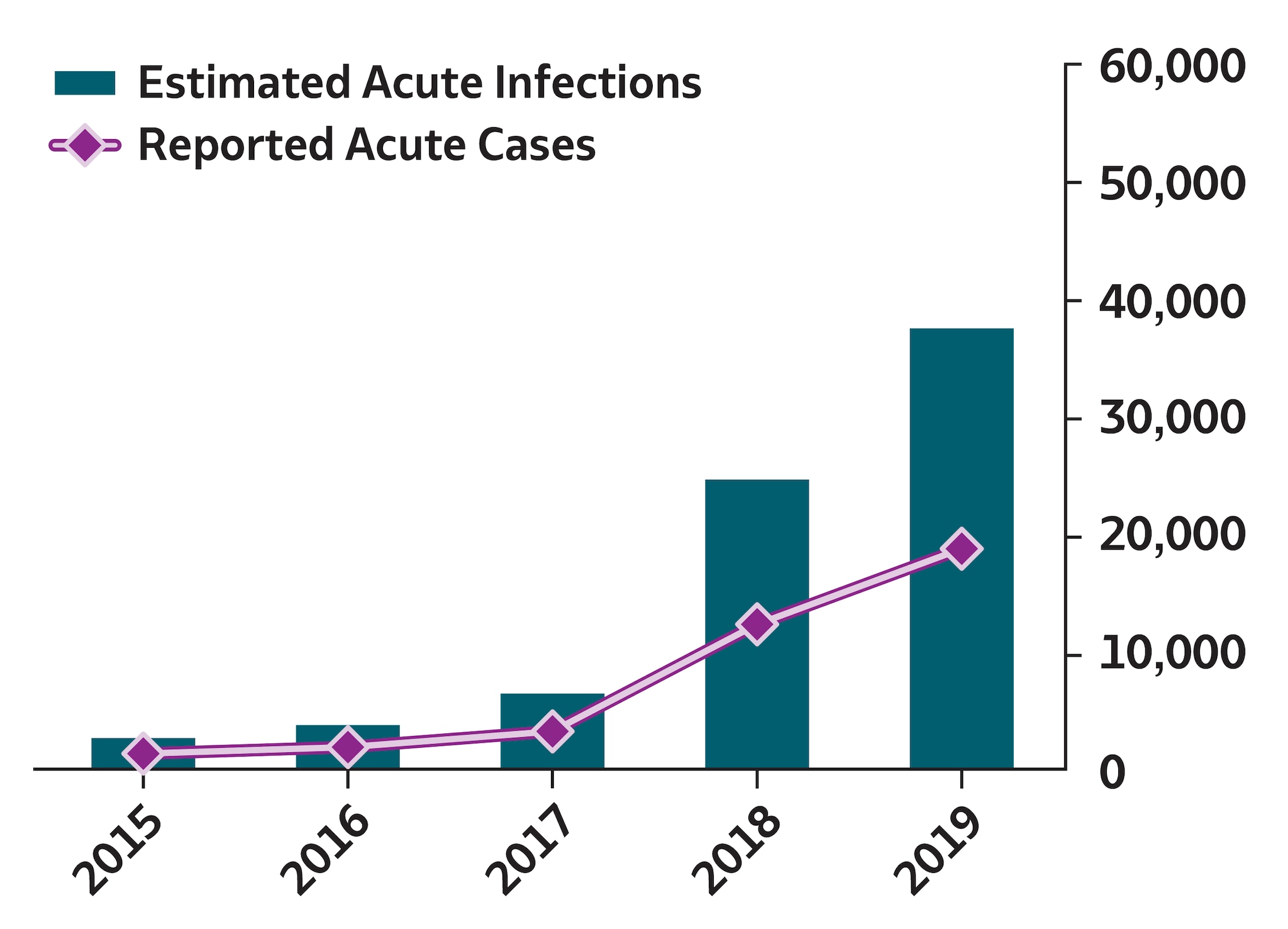
During 2019, a total of 18,846 hepatitis A cases were reported to CDC, corresponding to 37,700 estimated infections (95% confidence interval [CI]: 26,400–41,500) after adjusting for case underascertainment and underreporting (see Technical Notes) (9). The reported case count corresponds to a rate of 5.7 cases per 100,000 population, a 1,325% increase from the reported rate of 0.4 cases per 100,000 population during 2015. This increase was primarily driven by widespread person-to-person outbreaks of hepatitis A that have been unprecedented since introduction of the hepatitis A vaccine. These outbreaks are primarily occurring among persons who use drugs and those experiencing homelessness, resulting in prolonged community outbreaks in multiple states (10) that have been difficult to control. Approximately 75% of hepatitis A cases reported to CDC during 2019 occurred among persons aged 20–49 years, and 73% occurred among non-Hispanic White persons. Among the 10,991 (58%) reported cases that included risk information for injection drug use, 5,017 (46%) reported injection drug use. A total of 9,380 patients were hospitalized (64% hospitalization rate among the 14,619 cases with hospitalization information available).
Data from death certificates filed in the vital records offices of the 50 states and the District of Columbia revealed that the age-adjusted death rate associated with hepatitis A during 2019 among US residents was 0.04 deaths per 100,000 population, which is 4 times the rate of 0.01 deaths per 100,000 population during 2015.
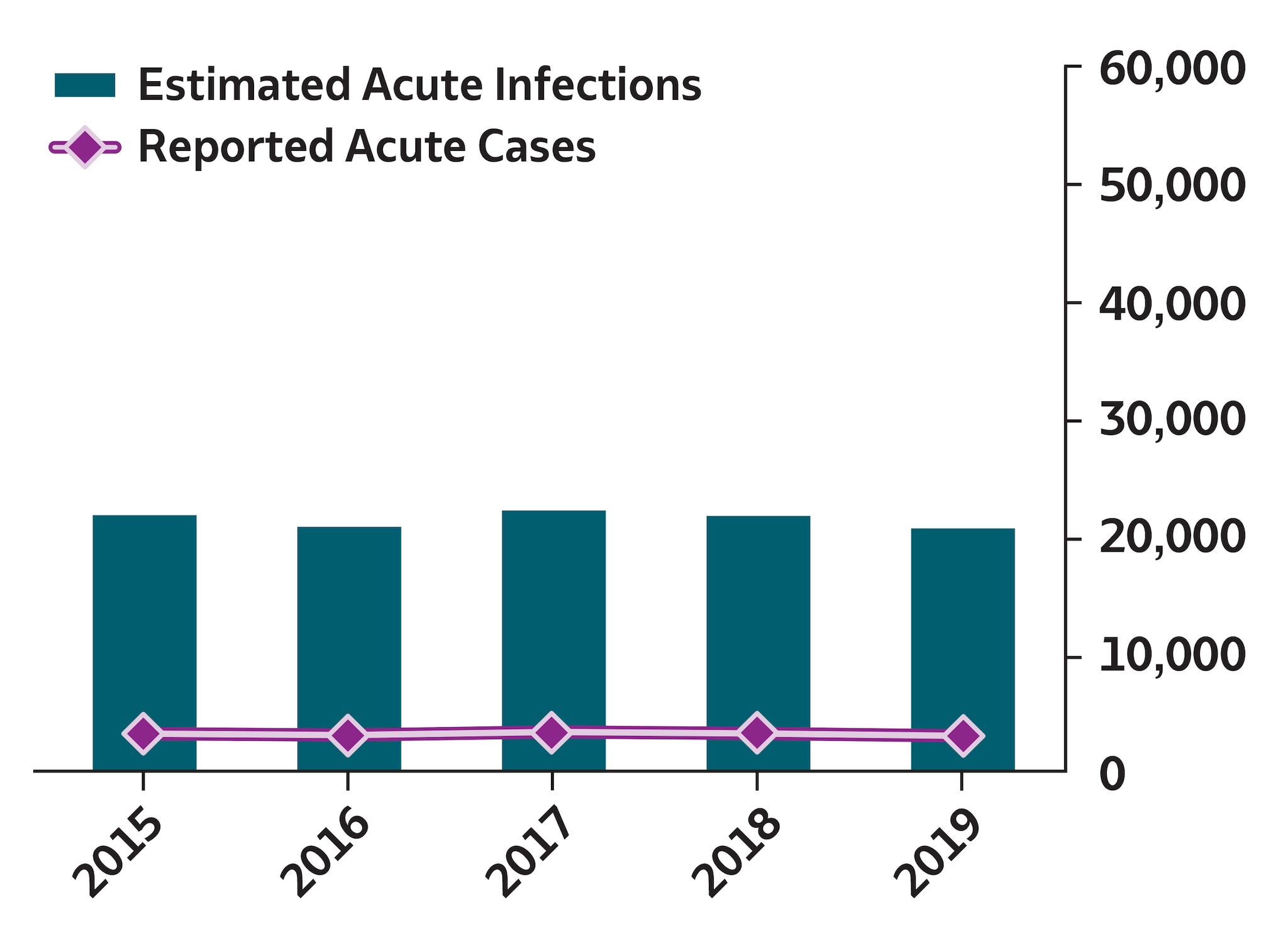
Reported cases of acute hepatitis B virus infection decreased after routine vaccination of children was recommended in 1991, and the number of cases became relatively stable during 2010–2019. During 2019, a total of 3,192 acute hepatitis B cases were reported to CDC, resulting in 20,700 estimated infections (95% CI: 11,800–50,800) after adjusting for case underascertainment and underreporting (see Technical Notes) (9). The reported case count corresponded to a rate of 1.0 per 100,000 population. Approximately 80% of acute hepatitis B cases reported to CDC during 2019 occurred among persons aged 30–59 years. The rate of acute hepatitis B was highest among non-Hispanic White persons (1.0 case per 100,000 population), compared with other racial/ethnicity groups. Among the 1,780 (56%) reported cases that included risk information for injection drug use, 631 (35%) reported injection drug use. A total of 1,427 patients with acute hepatitis B were hospitalized (64% hospitalization rate among 2,234 cases with hospitalization information available).
A total of 13,859 new cases of chronic hepatitis B were reported to CDC during 2019, corresponding to a rate of 5.9 cases per 100,000 population; 47% occurred among persons aged 30–49 years. The rate of new chronic hepatitis B was highest among Asian/Pacific Islander persons (18.9 cases per 100,000 population), which was >10 times the rate among non-Hispanic White persons (1.8 cases per 100,000 population).
A total of 17 perinatal hepatitis B cases were reported through NNDSS to CDC during 2019. Among the 9,950 infants born during 2018 and managed by 64 jurisdictions in the Perinatal Hepatitis B Prevention Program (see Supplement), 97% had received recommended prophylaxis at birth; 87% had completed 3 doses of vaccine by age 12 months; and 69% had received recommended postvaccination serologic testing. Among those with postvaccination testing (6,828), 23 (0.3%) were cases of perinatal hepatitis B transmission.
Data from death certificates filed in the vital records offices of the 50 states and the District of Columbia demonstrated that the age-adjusted death rate associated with hepatitis B during 2019 among US residents was 0.42 deaths per 100,000 population, approximately the same as the rate of 0.43 deaths per 100,000 population during 2018.
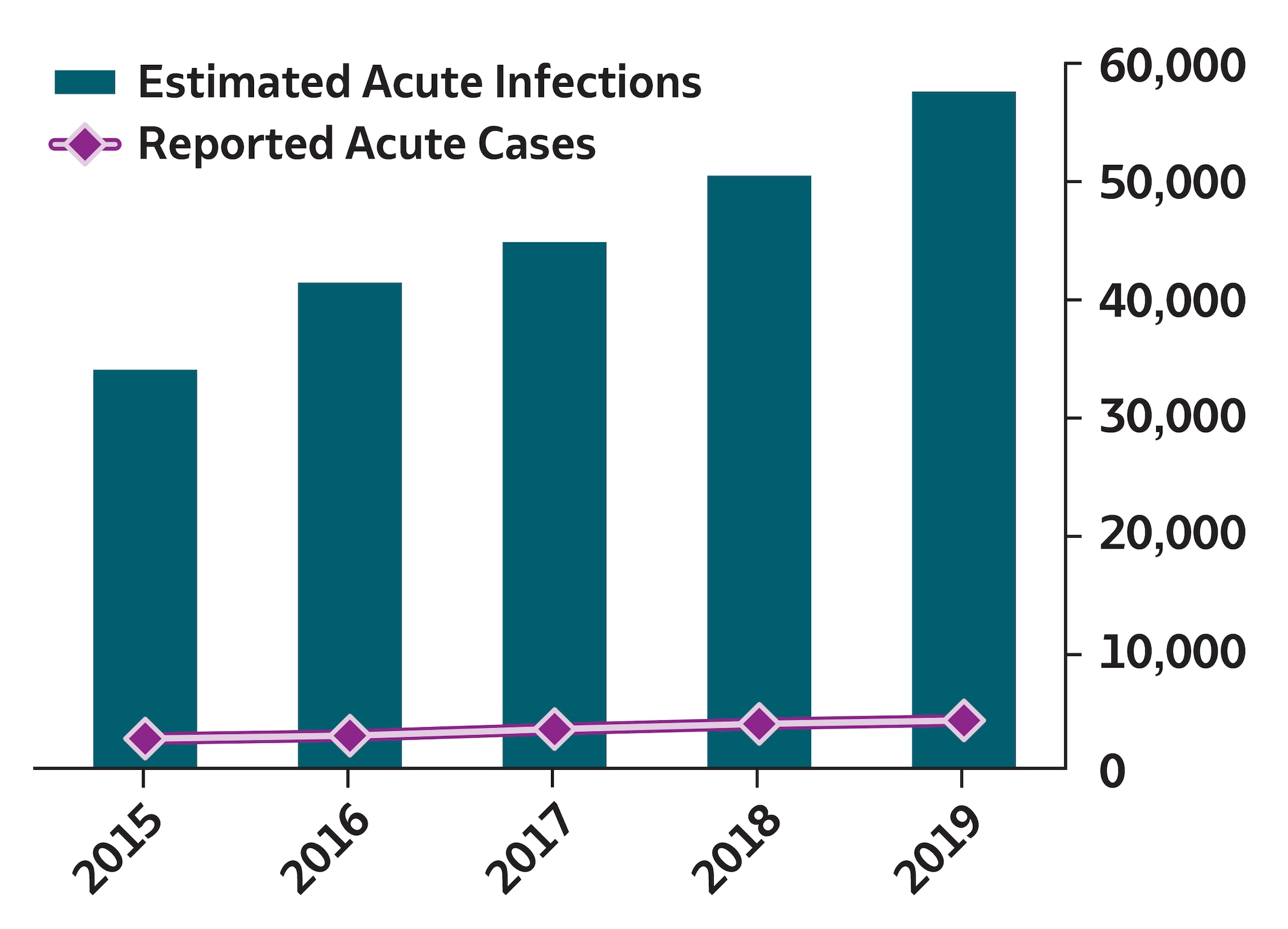
During 2019, a total of 4,136 acute hepatitis C cases were reported to CDC, corresponding to 57,500 estimated infections (95% CI: 45,500–196,000) after adjusting for case underascertainment and underreporting (see Technical Notes) (9). The reported acute hepatitis C case count corresponds to a rate of 1.3 cases per 100,000 population, a 63% increase from the reported rate of 0.8 cases per 100,000 population during 2015. Approximately 63% of acute hepatitis C cases reported to CDC during 2019 were among persons aged 20–39 years. The rate of acute hepatitis C was highest among American Indian/Alaska Native persons (3.6 cases per 100,000 population), compared with other racial/ethnicity groups. Among the 1,952 (47%) reported acute cases that included risk information for injection drug use, 1,302 (67%) reported injection drug use. A total of 1,041 patients with acute hepatitis C were hospitalized (48% hospitalization rate among 2,156 cases with hospitalization information available).
A total of 123,312 new cases of chronic hepatitis C were reported to CDC during 2019, corresponding to a rate of 56.7 cases per 100,000 population. The rate of newly reported chronic hepatitis C was highest among persons aged 30–39 years (109.1 cases per 100,000 population), followed by persons aged 50–59 years (79.6 cases per 100,000 population), compared with other age categories. These rates are consistent with the previously reported bimodal distribution of newly reported chronic hepatitis C affecting multiple generations (11). The rate of newly reported chronic hepatitis C cases was highest among American Indian/Alaska Native persons (86.7 cases per 100,000 population), compared with other racial/ethnicity categories.
A total of 217 perinatal hepatitis C cases were reported to CDC during 2019, the second year that standardized surveillance for perinatal hepatitis C was conducted by states and case notifications submitted to CDC. Data from death certificates filed in the vital records offices of the 50 states and the District of Columbia indicated that the age-adjusted death rate for hepatitis C during 2019 was 3.33 deaths per 100,000 population, representing a 32% decrease from the mortality rate during 2015 (4.91 deaths per 100,000 population).
TECHNICAL NOTES
Case Ascertainment and Case Reporting
For health department staff to report cases of viral hepatitis to CDC, systems and processes must be in place to ensure each case is detected. Because of varying state laws, resources, and infrastructure, not all health departments report all cases of acute or chronic viral hepatitis to CDC. Additionally, diagnosing every acute case is impossible, because symptoms might be either so mild that the person does not seek care or too vague to prompt a health care provider to suspect and test for viral hepatitis.
Case reporting begins when a local or state health department receives a positive laboratory report, indicating a person has a viral hepatitis infection. Because initial reporting provides limited information and clinical symptoms are frequently needed for classifying cases as acute, reported cases might require extensive follow-up to obtain full information for establishing case status and case classification.
Health departments prioritize cases for follow-up by using their own protocols and might submit cases to CDC with incomplete or missing information. Additionally, the volume of laboratory reports for chronic viral hepatitis infections might be so large that not all health departments are able to consistently detect and report all chronic cases to CDC; for example, during 2019, only 14 states (Florida, Georgia, Indiana, Kentucky, Louisiana, Massachusetts, New Jersey, North Carolina, Oklahoma, Ohio, Tennessee, Utah, Washington, and West Virginia) received federal funding for supporting viral hepatitis surveillance. Also, because case notifications for the 2019 reporting year were open for submission through December 10, 2020, the COVID-19 pandemic possibly affected a health department’s ability to investigate and report cases in its jurisdiction. Data regarding chronic hepatitis B and hepatitis C infections are included in this report where available; however, these are newly identified chronic viral hepatitis cases and do not measure prevalence.
All viral hepatitis conditions with no reported cases or characterized as Not Reportable or Data Unavailable for 2019 in a jurisdiction’s final signed report to CDC’s National Center for Surveillance, Epidemiology, and Laboratory Services (CSELS) were reported according to the following notation used by CSELS (12):
N : Not reportable. The disease or condition was not reportable by law, statute, or regulation in the reporting jurisdiction.
U : Unavailable. The data are unavailable.
For 2019 CSELS has additionally reported “The following 23 jurisdictions may have incomplete data, due to the coronavirus disease 2019 (COVID-19) pandemic: Alaska, California, Connecticut, District of Columbia, Florida, Idaho, Indiana, Kansas, Massachusetts, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New York (excluding New York City), New York City, North Dakota, Ohio, Oklahoma, South Carolina, Tennessee, Texas, and West Virginia.” (12).
Urbanicity: Urban and rural categorization was made according to CDC’s 2013 National Center for Health Statistics urban-rural classification scheme for counties and county-equivalent entities. Large central metropolitan, large fringe metropolitan, medium metropolitan, and small metropolitan counties were grouped as urban. Micropolitan and noncore counties were grouped as rural.
US Department of Health and Human Services regionsexternal icon provide a standardized structure for grouping jurisdictions into larger geographic areas. Ten regional offices directly serve state and local organizations.
| Region | Regional Office | State/Jurisdiction |
| 1 | Boston | Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont |
| 2* | New York | New Jersey, New York, Puerto Rico, Virgin Islands |
| 3 | Philadelphia | Delaware, District of Columbia, Maryland, Pennsylvania, Virginia, West Virginia |
| 4 | Atlanta | Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, Tennessee |
| 5 | Chicago | Illinois, Indiana, Michigan, Minnesota, Ohio, Wisconsin |
| 6 | Dallas | Arkansas, Louisiana, New Mexico, Oklahoma, Texas |
| 7 | Kansas City | Iowa, Kansas, Missouri, Nebraska |
| 8 | Denver | Colorado, Montana, North Dakota, South Dakota, Utah, Wyoming |
| 9* | San Francisco | Arizona, California, Hawaii, Nevada, American Samoa, Commonwealth of the Northern Mariana Islands, Federated States of Micronesia, Guam, Marshall Islands, Republic of Palau |
| 10 | Seattle | Alaska, Idaho, Oregon, Washington |
*US territories are not included in this report.
Case Definitions
To ensure consistent reporting across states, the Council for State and Territorial Epidemiologists, in collaboration with CDC, developed case definitions for viral hepatitis A, hepatitis B, and hepatitis C. The case definitions facilitate standardized reporting by using uniform criteria and differentiating between acute, chronic, and perinatal cases. When new technologies are developed for laboratory testing or better clinical data become available, the case definitions are updated. Changes in case definitions should be considered when examining temporal trends. For more information regarding 2019 case definitions, visit the National Notifiable Diseases Surveillance System’s internet site. No changes to case definitions were implemented for acute or chronic viral hepatitis during 2019.
Estimating Incidence of Acute Viral Hepatitis
To account for underascertainment and underreporting, a probabilistic model for estimating the true incidence of acute hepatitis A, hepatitis B, and hepatitis C from reported cases has been published previously (9). The model includes the probabilities of symptoms, referral to care and treatment, and rates of reporting to local and state health departments. The published multipliers have since been corrected by CDC to indicate that each reported case of acute hepatitis A represents 2.0 estimated infections (95% bootstrap CI: 1.4–2.2); each reported case of acute hepatitis B represents 6.5 estimated infections (95% bootstrap CI: 3.7–15.9); and each reported case of acute hepatitis C represents 13.9 estimated infections (95% bootstrap CI: 11.0–47.4).
Mortality Surveillance
The NVSS provides information regarding deaths that occur in the United States. NVSS data in this report are from the 2015–2019 Multiple Cause of Death files in the CDC WONDER online database (13). These data are based on information from all death certificates filed in the vital records offices of the 50 states and the District of Columbia through the Vital Statistics Cooperative Program. Deaths of nonresidents (e.g., nonresident aliens, nationals living abroad, or residents of US territories) and fetal deaths are excluded.
Perinatal Hepatitis B Prevention Program Surveillance
Outcome data regarding infants born to mothers with HBV infection are reported by the CDC Perinatal Hepatitis B Prevention Program. This program funds 64 jurisdictions to identify pregnant women infected with HBV and to case-manage their infants to improve receipt of postexposure prophylaxis, hepatitis B vaccine series completion, and postvaccination serologic testing. Data in this report are from the reporting period for the 2018 birth cohort, followed from January 1, 2018, through December 31, 2019, and only includes infants managed by the program. Infants have variable lengths of follow-up time, depending on their date of birth (https://www.cdc.gov/vaccines/programs/perinatal-hepb/index.html).
REFERENCES
- Nelson NP, Weng MK, Hofmeister MG, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep 2020;69(No. RR-5):1–38. doi: http://dx.doi.org/10.15585/mmwr.rr6905a1external icon
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67(No. RR-1):1–31. doi: http://dx.doi.org/10.15585/mmwr.rr6701a1external icon
- Centers for Disease Control and Prevention (CDC). Hepatitis B questions and answers for health professionals. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm
- Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018;108:175–81. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5846578/pdf/AJPH.2017.304132.pdfpdf iconexternal icon
- Centers for Disease Control and Prevention. Notes from the field: hepatitis C virus infections among young adults—rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep 2012;61:358. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6119a7.htm
- Seifert LL, Perumpail RB, Ahmed A. Update on hepatitis C: direct-acting antivirals. World J Hepatol 2015;7:2829–33. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4670954/pdf/WJH-7-2829.pdfpdf iconexternal icon
- Centers for Disease Control and Prevention (CDC). Division of Viral Hepatitis 2025 Strategic Plan, CDC; 2020. https://www.cdc.gov/hepatitis/pdfs/DVH-StrategicPlan2020-2025.pdfpdf icon
- Centers for Disease Control and Prevention. Viral Hepatitis Surveillance — United States, 2018. https://www.cdc.gov/hepatitis/statistics/2018surveillance/index.htm
- Klevens RM, Liu, S, Roberts H, et al. Estimating acute viral hepatitis infections from nationally reported cases. Am J Public Health 2014;104:482. PMC3953761. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3953761/pdf/AJPH.2013.301601.pdfpdf iconexternal icon
- Centers for Disease Control and Prevention (CDC). Widespread person-to-person outbreaks of hepatitis A across the United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm
- Ryerson AB, Schillie S, Barker LK, et al. Vital signs: newly reported acute and chronic hepatitis C cases—United States, 2009–2018. MMWR Morb Mortal Wkly Rep 2020;69:399–404. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7147907/pdf/mm6914a2.pdfpdf iconexternal icon
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System, 2019 Annual Tables
of Infectious Disease Data. Atlanta, GA. CDC Division of Health Informatics and Surveillance.https://wonder.cdc.gov/nndss/nndss_annual_tables_menu.asp. - CDC WONDER dataset documentation and technical methods can be accessed at https://wonder.cdc.gov/nndss/nndss_annual_tables_menu.asp
SUMMARY 2019
Viral Hepatitis Acute Infections
Reported in 2019

(26,400 – 41,500)*
Estimated in 2019

Reported in 2019

(11,800 – 50,800)*
Estimated in 2019

Reported in 2019

(45,500 – 196,000)*
Estimated in 2019
*95% Bootstrap Confidence Interval