Technical Update for HIV Nucleic Acid Tests Approved for Diagnostic Purposes
The Centers for Disease Control and Prevention (CDC) and the Association of Public Health Laboratories (APHL) are sharing information on three HIV nucleic acid tests (NATs) approved by the U.S. Food and Drug Administration (FDA) for diagnostic use: the cobas® HIV-1/HIV-2 Qualitative test (“HIV-1/HIV-2 Qualitative test,” approved August 2020, Roche Molecular Systems Inc., Branchburg NJ), the Aptima® HIV-1 Quant Dx Assay (approved November 2020, Hologic Inc., San Diego, CA), and the Alinity m HIV-1 Assay (“HIV-1 Assay,” approved July 2022, Abbott Molecular, Inc., Des Plaines, IL); the latter two assays provide both a qualitative and quantitative result. This document provides guidance on these HIV NATs with a diagnostic claim in the third step of the current recommended algorithm for laboratory testing (see Figure 1 below). When testing for the purpose of PrEP initiation similar principles apply. Additional information is provided regarding interpretations and considerations for when a HIV NATs with a diagnostic claim might be requested as part of a test sequence that differs from the current recommended algorithm.
CDC and APHL have not changed the recommendation that laboratories perform an HIV-1/HIV-2 antibody differentiation supplemental immunoassay as the second step after a reactive antigen/antibody screening immunoassay [1,2]. Figure 1 shows the update to the recommended algorithm for the diagnosis of HIV infection in which the recently FDA-approved HIV NATs with a diagnostic claim are incorporated at the third step. In situations when the differentiation assay is negative or indeterminate for HIV-1 or HIV-2 antibodies, the laboratories should perform a NAT with a diagnostic claim as a third test [3,4]. Algorithm interpretations at decision endpoints are shown in boxes. The vast majority (99.94%) of all reactive HIV screening tests performed in the US are resolved to be HIV-1 infections [5-7].
Diagnostic applications
All three of these HIV NATs are intended to be used as an aid in the diagnosis of HIV infection. The HIV-1/HIV-2 Qualitative test detects the presence of HIV-1 and/or HIV-2 RNA, whereas the HIV-1 Quant Dx Assay and the HIV-1 Assay detect the presence of HIV-1 RNA. The intended use of the HIV-1/HIV-2 Qualitative test also extends to HIV-2 confirmation. The detection of HIV-1 or HIV-2 nucleic acid is indicative of HIV-1 or HIV-2 infection, respectively. Detection of HIV-1 or HIV-2 nucleic acid in the absence of HIV antibodies is indicative of acute HIV-1 or HIV-2 infection. As a reminder, quantitative NATs (viral load assays) are not approved by the FDA for diagnosis unless the test has a dual claim which allows it to be used for both the diagnosis of HIV-1 infection (i.e., qualitative NAT) and for the clinical management of HIV-1 infection (i.e., quantitative NAT). Currently, only the HIV-1 Quant Dx Assay and the HIV-1 Assay have this dual claim.
Results and interpretations for NATs used for diagnostic purposes
The NAT used for diagnostic purposes will depend on the assay available at the laboratory performing the testing. While there is no difference in the capacity to diagnose HIV-1 with either NAT assay, there are a few differences among the assays to note that may be considered in determining which test may be most appropriate in extenuating circumstances, such as likelihood of HIV-2 infection.
The HIV-1/HIV-2 Qualitative test is presently the only FDA-approved qualitative NAT that provides and differentiates results for HIV-1 and HIV-2; this includes an overall result, individual results for HIV-1 and HIV-2, and a result interpretation for serum and plasma. Possible valid result combinations for the HIV-1/HIV-2 Qualitative test and interpretation are represented in the following table:
following table:
| HIV-1/HIV-2 Qualitative Test Result
(serum and plasma) |
|
| Overall Result | Interpretation |
| Overall result: “Reactive”
Target results: HIV-1 reactive and HIV-2 reactive |
Target signal detected for HIV-1 and HIV-2 |
| Overall result: “Reactive”
Target result: HIV-1 reactive and HIV-2 non-reactive |
Target signal detected for HIV-1. No target signal detected for HIV-2 |
| Overall result: “Reactive”
Target result: HIV-1 non-reactive and HIV-2 reactive |
No target signal detected for HIV-1. Target signal detected for HIV-2 |
| Overall result: “Non-Reactive” Target result: HIV-1 non-reactive and HIV-2 non-reactive | No target signal detected for HIV-1 or HIV-2 |
To clarify results for HIV prevention and surveillance grantees, and HIV testing and care providers, laboratories should report, at a minimum, the overall result and the individual target results. If there is concern that a patient has exposure to HIV-2 or there is evidence of HIV-2 antibody reactivity and the initial NAT used was an HIV-1 only test, a NAT that detects HIV-2 might be warranted.
For the HIV-1 Quant Dx Assay, the “Dx” designation is due to the assay being approved for diagnostic use with serum and plasma specimens. Laboratories must use the included instructions for use (IFU) to interpret the HIV-1 RNA concentration as a qualitative result and follow the “User’s Diagnostics Qualitative Interpretation,” summarized in the below table. If plasma is utilized, the quantitative result is valid and may be reported, but serum may not be used to obtain quantitative results.
| HIV-1 Quant Dx Assay | ||
| System Reported | User Performed | |
| Result
(copies/mL)** |
Interpretation | Confirmatory Interpretation |
| Not detected* | Target not detected | Non-reactive for HIV-1 RNA |
| <30 | Detected <30 | Reactive for HIV-1 RNA |
| 30 to 10,000,000 | Detected and quantified | Reactive for HIV-1 RNA |
| >10,000,000 | >10,000,000 | Reactive for HIV-1 RNA |
* A “Not detected” result should not be reported with a numerical value (e.g., <30) to avoid confusion in conveying the absence or presence of detectable HIV-1 RNA.
**Quantitative results can be reported on plasma samples only.
The HIV-1 Assay was recently approved for diagnostic interpretation as a supplemental test in addition to its use to monitor the progression of HIV-1 infection. For plasma samples, laboratories must use the included instructions for use (IFU) to interpret the HIV-1 RNA concentration as a qualitative result by following the “User’s Diagnostics Qualitative Interpretation,” summarized in the below table.
| HIV-1 Assay
(plasma) |
|||
| System Reported | User Performed | ||
| Result
(copies/mL) |
Interpretation | Confirmatory Interpretation | |
| Not detected* | Target not detected | Negative | |
| <20 | Detected <20 | Positive | |
| 20 to 10,000,000 | Detected and quantified | Positive | |
| >10,000,000 | >10,000,000 | Positive | |
* A “Not detected” result should not be reported with a numerical value (e.g., <20) to avoid confusion in conveying the absence or presence of detectable HIV-1 RNA.
For serum samples, quantitative results are not reported. Instead, only the qualitative results are reported as summarized in the table below.
| HIV-1 Assay
(serum) |
|
| Alinity m System Reported | |
| Result | Interpretation |
| HIV-1 RNA not detected | Negative |
| HIV-1 RNA detected | Positive |
Quantitative results for both the HIV-1 Quant Dx Assay and the HIV-1 Assay that are below the lower limit of quantification can cause some confusion. The qualitative interpretation of HIV-1 RNA concentrations is especially important when the result obtained is detected, but not quantifiable. This result means that HIV-1 RNA has been detected but the quantitative value was below the limit of quantification of the test (20 or 30 copies/mL) meaning that the amount of RNA cannot be reliably measured. It is important to report these results as “Reactive for HIV-1 RNA” (i.e., HIV-1 RNA is detected) [8]. HIV infections with low HIV-1 RNA levels before seroconversion have been described for elite suppressors [9] or persons taking antiretrovirals, including PrEP. Considerations for testing persons prescribed PrEP are provided in a separate guidance [10] and addressed below.
Figure 1. Recommended HIV diagnostic algorithm updated to include currently approved HIV-1 and HIV-1/-2 NATs with a diagnostic claim for the third step.
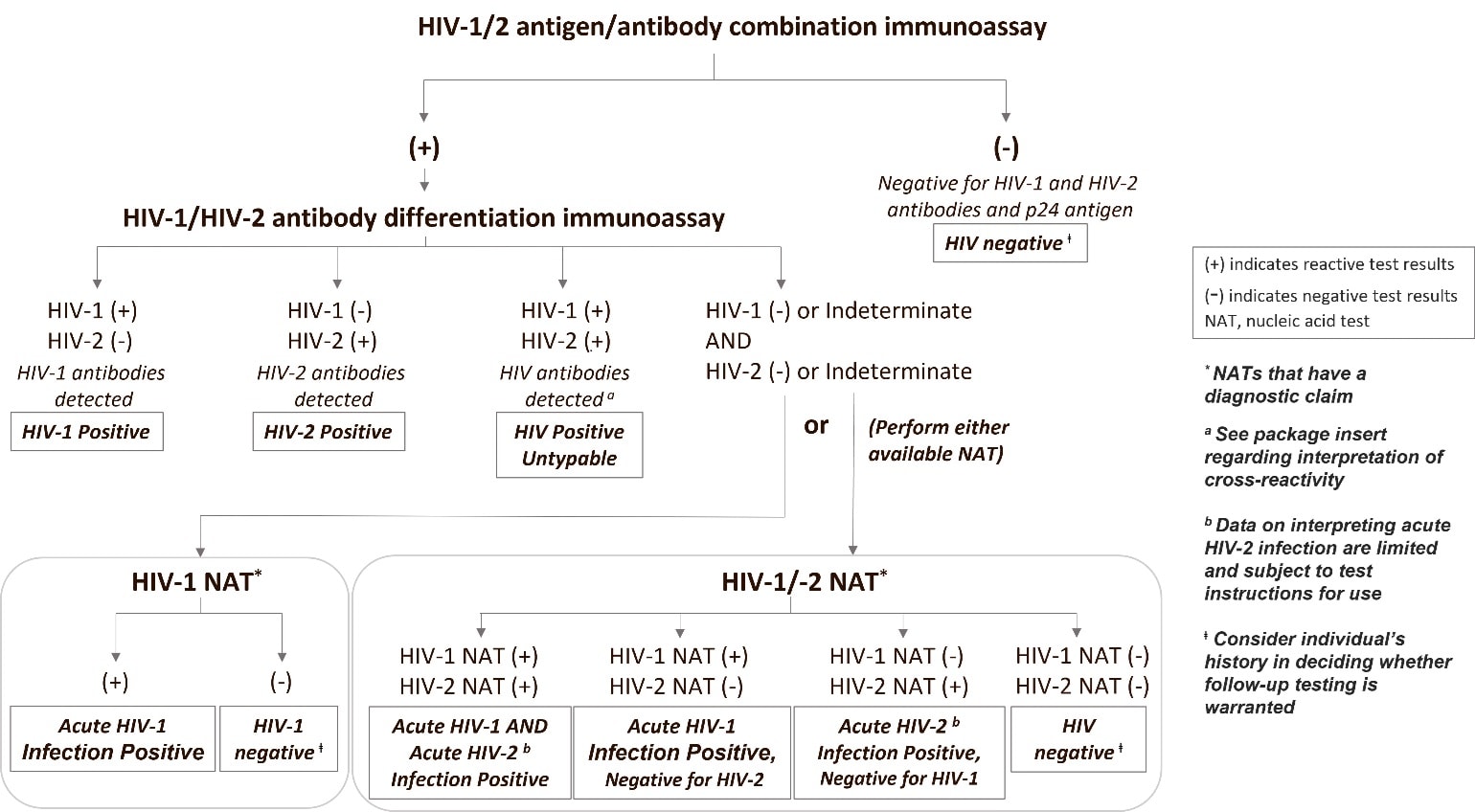
Nucleic acid tests have high sensitivity for detecting HIV infection in persons not taking antiretrovirals. For specimens with confirmed detectable HIV RNA, the sensitivity of each nucleic acid test is >99%, meaning that HIV is detected more than 99% of the time. However, under certain circumstances a negative test result does not necessarily mean that a person is not infected with HIV; it is important to remember that the detection of HIV-1 and HIV-2 nucleic acid is dependent on the number of virus particles present in the sample. From a clinical perspective, an important consideration for any negative (non-reactive) test result is whether virus replication has reached a level that can be detected. For example, test results are not reliable when an individual is tested during the eclipse period. The eclipse period is the time from the onset of HIV infection until virus is detectable by virologic tests; this time varies in duration from 10 days to as long as 33 days. Additionally, if HIV infection has occurred, the use of either prescribed or off-label use of pre-exposure prophylaxis (PrEP) will result in low, and possibly undetectable, serum and plasma concentrations of HIV RNA [11,12]. Moreover, early treatment of infection or transmission that leads to defective HIV replication or non-progression of disease may result in low virus expression. These situations may impact the detection capacity of both serologic and nucleic acid tests due to low virus levels and delayed or inhibited seroconversion. Other reasons for false negative test results include technical issues such as incorrect specimen type (e.g., blood collected in tube with an incompatible preservative), specimen mix-up, improper specimen storage conditions, and mislabeling or data transcription errors.
Use of HIV-1/HIV-2 differentiation NAT following an HIV-1/HIV-2 antibody differentiation supplemental assay
The ability to detect and differentiate HIV-1 from HIV-2 RNA as a third step will be useful in cases where the supplemental antibody test was unable to differentiate and/or confirm HIV-1/HIV-2 infections. For example, results from the Geenius™ HIV 1/2 Supplemental Assay (Bio-Rad Laboratories, Redmond, WA) can be HIV-2 indeterminate, HIV-1 indeterminate, HIV indeterminate, HIV positive untypable (undifferentiated), or HIV antibody negative [4-7, 13,14]. Likewise, results from the VioOne HIV Profile Supplemental Assay (Avioq Inc, Research Triangle Park, NC) can be HIV-1 indeterminate or HIV negative [2]. In all these situations, an HIV NAT with a diagnostic claim should be conducted as a third step. Nucleic acid testing may also be useful in situations where the conclusion from the second step is untypable. The specific HIV NAT used in the third step impacts the final algorithm interpretation and recommended next steps. An HIV-1 only NAT allows confirmation of HIV-1 infection and may indicate additional follow-up is needed if the previous tests have not ruled out HIV-2 infection. A NAT that detects and differentiates HIV-1 and HIV-2 RNA can simultaneously confirm either or both HIV-1 and HIV-2 infection (Figure 1). If the HIV-1/HIV-2 qualitative test is non-reactive, there is no laboratory evidence of HIV infection. However, if recent infection, PrEP use, or technical testing issues are known or suspected, additional HIV testing should be conducted to conclusively rule out HIV infection [10].
The antibody supplemental assays may provide results that are HIV-2 positive with HIV-1 cross-reactivity or HIV-1 positive with HIV-2 cross-reactivity. The instructions for use indicate that those results should be interpreted as HIV-2 positive and HIV-1 positive, respectively. However, there is a rare possibility of dual infection, so if a potential HIV-2 infection is suspected because of an individual’s known exposure to an HIV-2 infected person or travel history to or residence in an HIV-2 endemic area (primarily West African nations), the infection status should be resolved with an HIV-1/HIV-2 Qualitative test that differentiates HIV-1 and HIV-2 RNA. Alternatively, sequential HIV-1 and HIV-2 NATs could be performed.
Testing sequences when HIV NAT with a diagnostic claim is alternatively ordered as the second step in an algorithm
While it is currently not recommended as the standard laboratory algorithm, in certain circumstances, such as symptoms coinciding with a known or suspected recent exposure, an alternative testing sequence in which NAT with a diagnostic claim is applied in the second step may be ordered by a health care provider. This may accelerate detection of acute HIV infection and be beneficial to timely clinical decision-making. Additionally, in rare situations where individuals have participated in vaccine or neutralizing antibody prevention trials, antibody results may not be reliable indicators of infection. Finally, considerations for testing persons prescribed PrEP are provided in a separate guidance and include screening with both an antibody test and a NAT [10]. Below we describe algorithm interpretations and information related to when a NAT with a diagnostic claim might be used in the second step of the diagnostic algorithm rather than using the recommended antibody differentiation assay. [15]
Figure 2. Testing sequence when using an HIV differentiation NAT with diagnostic claim as a second step in the diagnostic algorithm.
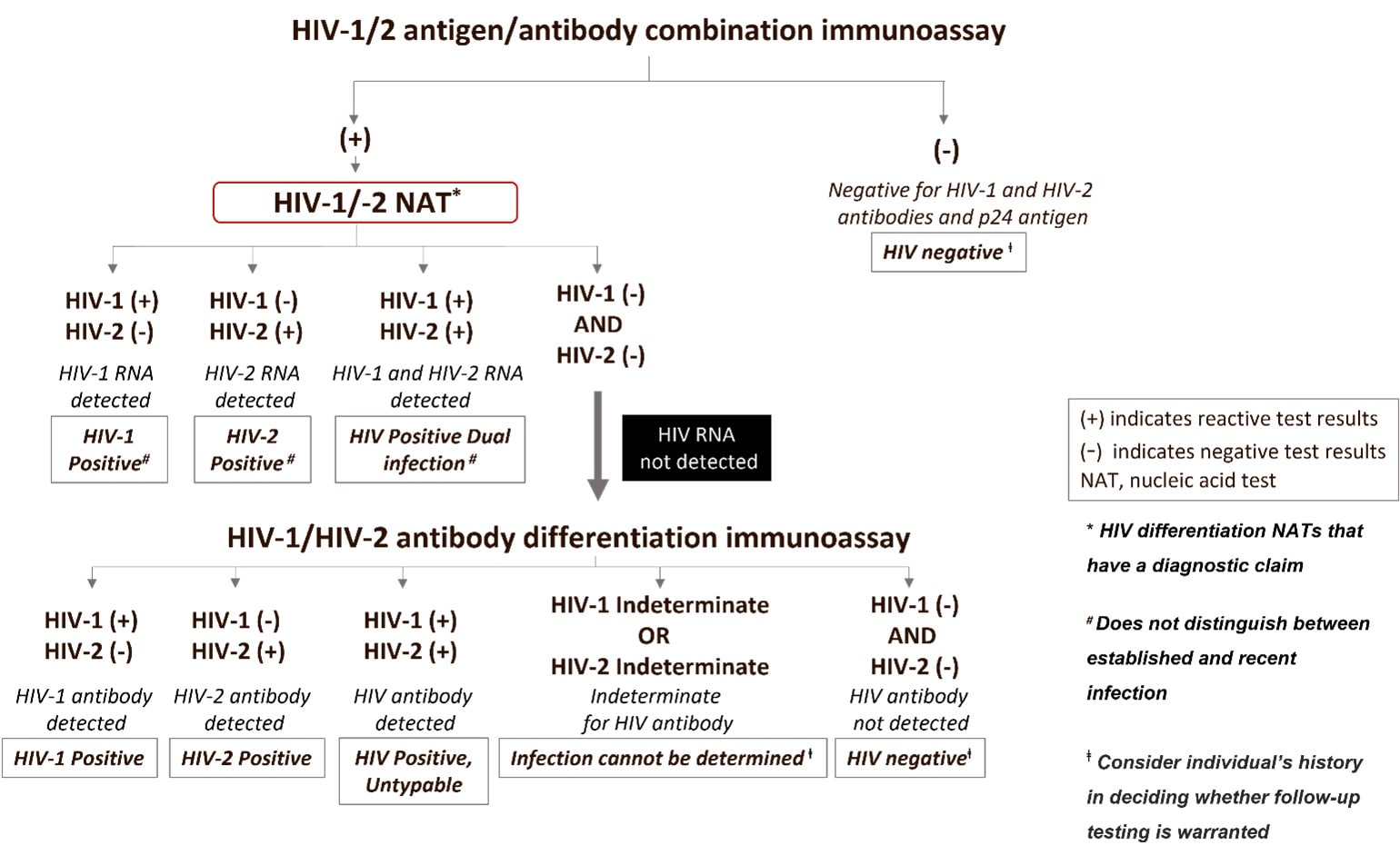
Figure 3. Testing sequence when using an HIV-1 NAT with a diagnostic claim as the second step in the algorithm.
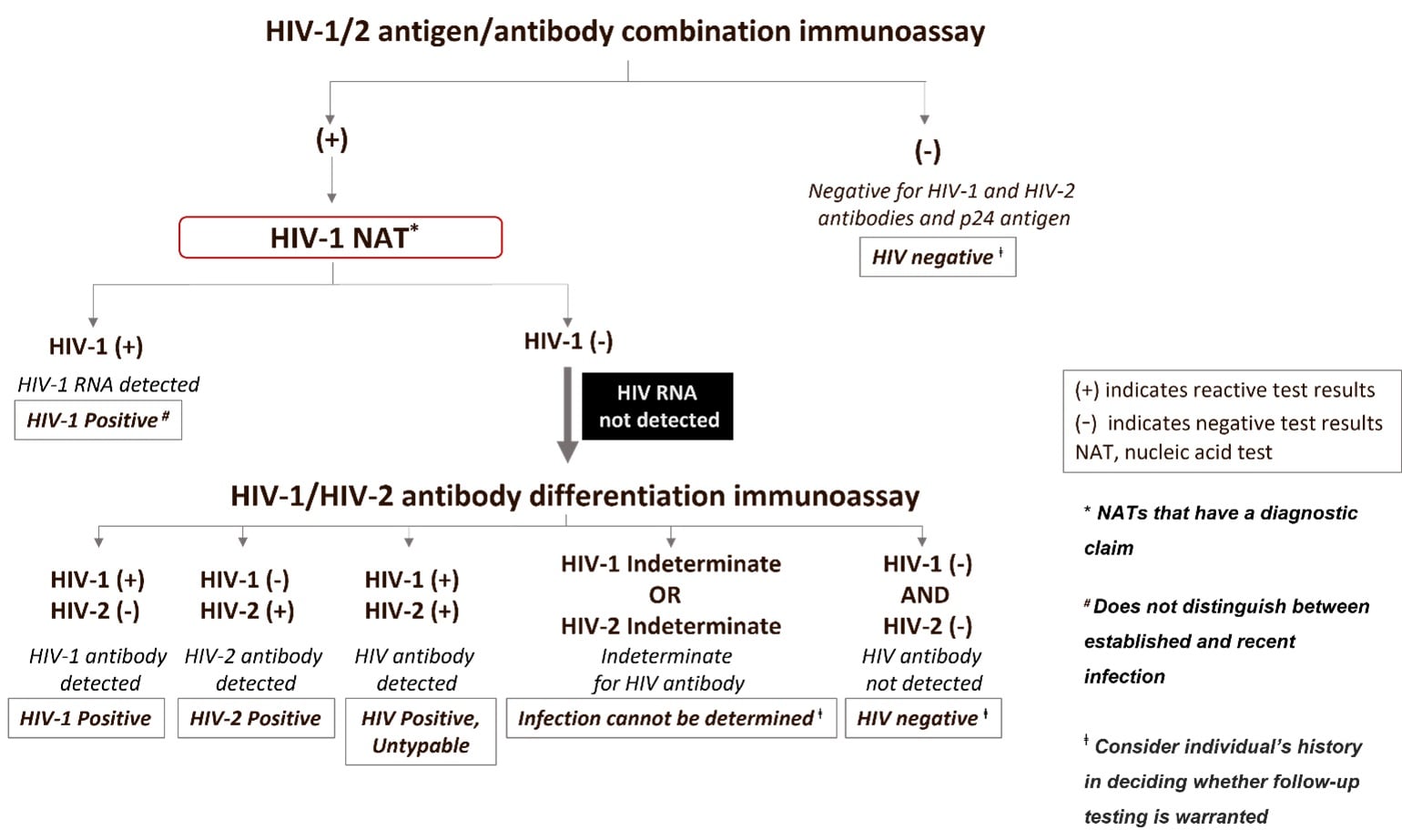
The interpretations and caveats of using a NAT for diagnostic purposes in the second step of the testing sequence will differ depending on whether the NAT can detect both HIV-1 and HIV-2 RNA (figure 2) or HIV-1 RNA only (figure 3). In these circumstances, interpretations following a reactive antigen/antibody combination or a reactive antigen immunoassay result, include:
| Result of a NAT with a Diagnostic Claim Used at the Second Step | Test Follow-up | Interpretation |
| HIV-1 or HIV-2 NAT Reactive | No additional testing needed | Infection with HIV-1 or HIV-2, respectively |
| HIV-1 or HIV-2 NAT Non-reactive | Perform follow-up testing with an HIV-1/HIV-2 antibody differentiation supplemental assay | HIV-1/HIV-2 antibody negative, result is evidence of a false-reactive initial test when testing in the absence of antiretroviral therapy.
HIV-1/HIV-2 antibody indeterminate, this result is expected to be extremely rare, follow up testing, e.g., DNA NAT or another specimen draw, is recommended to confirm infection status.* HIV-1/HIV-2 antibody positive, result is indicative of HIV infection. |
*CDC only has limited data when using the differentiation assay in this sequence of testing. Therefore, because of the current data limitation we are indicating infection status cannot be determined from this testing sequence. For individuals enrolled in vaccine trials, an indeterminate result would indicate the individual is likely not infected. Additional follow-up may be needed if recent behavior associated with increased HIV acquisition is documented.
It is expected that an indeterminate result for the HIV antibody differentiation assay at the third step will be rare and could be due to persons with HIV who are ARV-experienced; follow up testing is recommended as a test sequence should not end with an indeterminate result. ARV experience, particularly when ART is started early or with long-term viral suppression, could affect any test result and would pertain to any sequence of tests. If confirmation of infection status is needed, then additional testing is recommended.
In addition to the limitations mentioned above which presently prevent recommending systemic use of a NAT with a diagnostic claim as the second step, there are other limitations of the alternative algorithm to note. Using a NAT with a diagnostic claim at the second step to confirm infection will not be able to distinguish acute HIV infection from established HIV infection unless a screening assay that distinguishes Ag from Ab reactivity is used in the first step. In this case, an Ag-only reactive test result followed by a reactive a NAT with a diagnostic claim would be evidence of acute HIV infection. The use of a NAT with a diagnostic claim in the alternative algorithm may be cost-prohibitive for some laboratories. This approach may incur increased cost and turnaround time if the NAT used has been validated as a diagnostic test, as additional testing will be required. Turn-around time can also be lengthened for lower-throughput laboratories if samples must be batched for conservation of reagents. Impact of performing HIV NAT with a diagnostic claim in the second step on surveillance: Early (stage 0) and acute HIV infections
Use of an HIV NAT with a diagnostic claim as a second step in the testing algorithm will limit a health departments’ ability to identify and prioritize for public health follow-up persons with early (stage 0) HIV infection if the screening assay cannot differentiate antigen and antibody reactivity. Most infections classified as stage 0 are acute infection and are defined as having an absence of antibody reactivity [16]. For HIV surveillance, persons are classified as having stage 0 or acute infection based on a combination of discordant test results that include a negative or indeterminate HIV test result within 180 days (stage 0), or within 60 days (acute HIV), of the first positive HIV test result [17,18]. Acute HIV infection is typically identified by a positive HIV-1/2 Ag/Ab test result and a negative HIV-1/2 supplement antibody test result, followed by a reactive NAT as part of the HIV diagnostic testing algorithm. Without the HIV-1/2 supplemental antibody test as the second step in the algorithm, health departments would only infrequently have data available to identify possible acute HIV infection (e.g., clinical symptoms).
CLOSING
We reiterate that the current diagnostic algorithm, illustrated in Figure 1, is still the recommended sequence of testing. Any changes to the recommended algorithm require validation prior to updating testing recommendations. As evaluations of new tests and ongoing performance of existing assays continue to be assessed by CDC and other investigators, guidance will be updated as warranted by evidence that indicates an impact on diagnostic performance.
Laboratories should update their laboratory information systems to reflect accurate reporting results from NATs with a diagnostic claim and update LOINC assignments (HIV test LOINC map).
There will be cases for which diagnostic tests and algorithms do not yield an accurate result. Biologic causes for false-positive and false-negative HIV test results have been reported [19]. Attention to pre- and post-analytic steps to alleviate incorrect specimen type, incorrect shipping or storage, specimen mix-up, mislabeling or data transcription errors will help to minimize incorrect test results. Additionally, awareness of clinical conditions, such as autoimmune disease and parasitic infections, can help to resolve false serologic reactivity that may not be confirmed by a NAT.
Please send any comments or questions to www.cdc.gov/info or 1-800-CDC-INFO.
References:
- Bio-Rad Laboratories. Geenius HIV 1/2 Supplemental Assay instructions for use. 2014; http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/UCM420735.pdf.
- VioONE HIV Profile Supplemental Assay package insert rev 5. 2020; https://www.fda.gov/media/143115/download
- Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. 2014. http://stacks.cdc.gov/view/cdc/23447. Accessed June 1, 2021.
- APHL Suggested Reporting Language for HIV Laboratory Diagnostic Testing Algorithm. 2019; https://www.aphl.org/aboutAPHL/publications/Documents/ID-2019Jan-HIV-Lab-Test-Suggested-Reporting-Language.pdf.
- Peruski AH, Wesolowski LG, Delaney KP, Chavez PR, Owen SM, Granade TC, Sullivan V, Switzer WM, Dong X, Brooks JT, Joyce MP. Trends in HIV-2 Diagnoses and Use of the HIV-1/HIV-2 Differentiation Test – United States, 2010-2017. MMWR Morb Mortal Wkly Rep. 2020 Jan 24;69(3):63-66. doi: 10.15585/mmwr.mm6903a2. PMID: 31971928; PMCID: PMC7367036.
- Wesolowski LG, Chavez PR, Cárdenas AM, Katayev A, Slev P, Valsamakis A, Wang YF, Yao JD, Dougherty C, Gillim-Ross L, Harmon C, Delaney KP. Routine HIV Test Results in 6 US Clinical Laboratories Using the Recommended Laboratory HIV Testing Algorithm with Geenius HIV 1/2 Supplemental Assay. Sex Transm Dis. 2020. 47(5S Suppl 1):S13-S17. doi: 10.1097/OLQ.0000000000001102. PMID: 32343517.
- Delaney K. Evaluation of newly approved HIV antigen-antibody tests individually and when used in the CDC/APHL HIV diagnostic algorithm. HIV Diagnostics Conference; 2016; Atlanta, GA.
- Use and Interpretation of Quantitative HIV-1 RNA Test Results: Guidance for Laboratories; https://www.aphl.org/aboutAPHL/publications/Documents/ID_2021_HIV-1_RNA_Test_Reporting_Guide.pdf.
- Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC, Baker B, Rosenberg R, Cutrell E, Seaman MS, Coffin JM, Walker BD. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009 Sep 15;200(6):984-90. doi: 10.1086/605446.
- Centers for Disease Control and Prevention: US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 Update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf.
- Sivay MV, Li M, Piwowar-Manning E, et al. Characterization of HIV seroconverters in a TDF/FTC PrEP study: HPTN 067/ADAPT. J Acquir Immune Defic Syndr. 2017;75(3):271.
- Donnell D, Ramos E, Celum C, et al. The effect of oral preexposure prophylaxis on the progression of HIV-1 seroconversion. AIDS. 2017;31(14):2007.
- Luo W. Performance Evaluation of HIV Supplemental Assays. HIV Diagnostics Conference; 2016; Atlanta, GA.
- Fordan S. Comparative Performance of the Geenius HIV-1/HIV-2 Supplemental Test in Florida’s Public Health Testing Population. HIV Diagnostics Conference; 2016; Atlanta, GA.
- Masciotra S, Luo Wei, Rossetti R, Smith T, Ethridge S, Delaney K, Wesolowski L, Owen SM. Could HIV-1 RNA Testing be an Option as the Second Step in the HIV Diagnostic Algorithm? Sex Transm Dis. May 2020. 47(5S):S26-S31 doi: 10.1097/OLQ.0000000000001137.
- Linley L, Selik RM, Delaney KP, Oster AM. Improved Detection of Acute HIV in the United States, 2012-2017. 26th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, March 4-7, 2019.
- Selik RM, Mokotoff ED, Branson B, Owen SM, Whitmore S, Hall, HI. MMWR 2014;63(No. RR-3):1-10. Revised Surveillance Case Definition for HIV Infection — United States, 2014.
- Selik RM, Linley L. Viral loads within 6 weeks after diagnosis of HIV infection in early and later stages: Observational study using national surveillance data. JMIR Public Health Surveill; 2018;4(4):e10770. doi: 10.2196/10770.
- HIV False-positive HIV Test Results. May 2018; https://www.cdc.gov/hiv/pdf/testing/cdc-hiv-factsheet-false-positive-test-results.pdf