
Pneumocystis
[Pneumocystis jirovecii]
Causal Agents
Pneumocystis jirovecii (previously classified as Pneumocystis carinii) was previously classified as a protozoa. Currently, it is considered a fungus based on nucleic acid and biochemical analysis.
Reference:
- Frenkel JK. Pneumocystis pneumonia, an immunodeficiency-dependent disease (IDD): a critical historical overview. J Eukaryot Microbiol 1999;46:89S–92S.
- Stringer JR, Beard CB, Miller RF, Wakefield, AE. A New Name (Pneumocystis jiroveci) for Pneumocystis from Humans. Emerg Infect Dis 2002;8:891-896.
Life Cycle
Pneumocystis stages were reproduced from a drawing by Dr. John J. Ruffolo, South Dakota State University, USA published in Cushion M. Pneumocystis carinii. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson’s Microbiology and Microbial Infections: Volume 4 Medical Mycology, 9th ed. New York: Arnold Publishing; 1998. p. 674. Copyright held by Arnold Publishing and reproduced here by permission of Arnold and Dr. Ruffolo.
Our thanks to Dr. Melanie T. Cushion for her comments on the life cycle text.
References:
- Ruffolo JJ. Pneumocystis carinii Cell Structure. In: Walzer, PD, editor. Pneumocystis carinii Pneumonia. 2nd ed. Marcel Dekker; 1994. p. 25-43.
- Cushion MT, Ruffolo JJ, Walzer PD. Analysis of the developmental stages of Pneumocystis carinii in vitro. Lab Invest 1988;58:324-331.
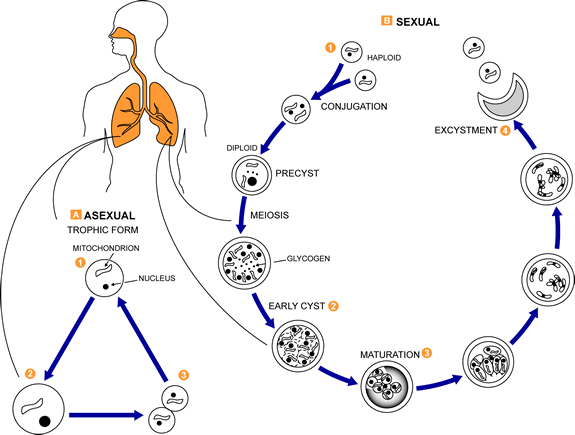
This is a generalized life cycle proposed by John J. Ruffolo, Ph.D. (Cushion, MT, 1988) for the various species of Pneumocystis. These fungi are found in the lungs of mammals where they reside without causing overt infection until the host’s immune system becomes debilitated. Then, an oftentimes lethal pneumonia can result. Asexual phase: trophic forms  replicate by mitosis
replicate by mitosis  to
to  . Sexual phase: haploid trophic forms conjugate
. Sexual phase: haploid trophic forms conjugate  and produce a zygote or sporocyte (early cyst)
and produce a zygote or sporocyte (early cyst)  . The zygote undergoes meiosis and subsequent mitosis to produce eight haploid nuclei (late phase cyst)
. The zygote undergoes meiosis and subsequent mitosis to produce eight haploid nuclei (late phase cyst)  . Spores exhibit different shapes (such as, spherical and elongated forms). It is postulated that elongation of the spores precedes release from the spore case. It is believed that the release occurs through a rent in the cell wall. After release, the empty spore case usually collapses, but retains some residual cytoplasm
. Spores exhibit different shapes (such as, spherical and elongated forms). It is postulated that elongation of the spores precedes release from the spore case. It is believed that the release occurs through a rent in the cell wall. After release, the empty spore case usually collapses, but retains some residual cytoplasm  . A trophic stage, where the organisms probably multiply by binary fission is also recognized to exist. The organism causes disease in immunosuppressed individuals.
. A trophic stage, where the organisms probably multiply by binary fission is also recognized to exist. The organism causes disease in immunosuppressed individuals.
Geographic Distribution
Worldwide, in humans and animals. Serologic evidence indicates that most healthy children have been exposed by age 3 to 4. Pneumocystis pneumonia (PCP) occurs in immunosuppressed individuals and in premature, malnourished infants.
Clinical Presentation
The symptoms of Pneumocystis pneumonia (PCP) include dyspnea, nonproductive cough, and fever. Chest radiography demonstrates bilateral infiltrates. Extrapulmonary lesions occur in a minority (<3%) of patients, involving most frequently the lymph nodes, spleen, liver, and bone marrow. Typically, in untreated PCP increasing pulmonary involvement leads to death.
Pneumocystis jirovecii trophozoites.

Pneumocystis jirovecii cysts.


Indirect immunofluorescence using monoclonal antibodies against Pneumocystis jirovecii.


Laboratory Diagnosis
The specific diagnosis is based on identification of P. jirovecii in bronchopulmonary secretions obtained as induced sputum or bronchoalveolar lavage (BAL) material. In situations where these two techniques cannot be used, transbronchial biopsy or open lung biopsy may prove necessary. Microscopic identification of P. jiroveci trophozoites and cysts is performed with stains that demonstrate either the nuclei of trophozoites and intracystic stages (such as Giemsa) or the cyst walls (such as the silver stains). In addition, immunofluorescence microscopy using monoclonal antibodies can identify the organisms with higher sensitivity than conventional microscopy.
Molecular Diagnosis
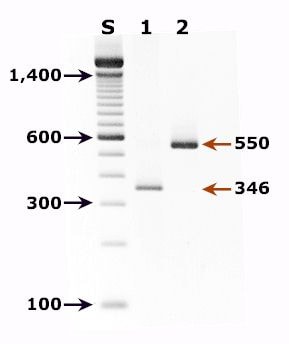
Agarose gel (2%) analysis of PCR-amplified products from DNA extracted from a broncho alveolar lavage (BAL) diagnostic specimen of a patient with pulmonary symptoms.
Molecular methods for detection of P. jirovecii have shown very high sensitivity and specificity and constitute the gold standard for detection of this pathogen.
Agarose gel (2%) analysis of PCR-amplified products from DNA extracted from a bronchoalveolar lavage (BAL) diagnostic specimen of a patient with pulmonary symptoms.
- Lane S: Molecular base pair standard (100-bp ladder). Black arrows show the size of standard bands.
- Lane 1: Single step PCR amplification with the pAZ102-E/pAZ102-H primer pair1 – diagnostic band size: 346 bp.
- Lane 2: Nested PCR amplification with the ITS nested PCR primers, 1724F/ITS2R (first round) and ITS1F/ITS2R1 (second round)2 – diagnostic band size: 550 bp.
References:
- Wakefield AE, Pixley FJ, Banerji S, Sinclair K, Miller RF, Moxon ER, Hopkin JM. Amplification of mitochondrial ribosomal RNA sequences from Pneumocystis carinii of rat and human origin. Mol Biochem Parasitol 1990;43:69-76.
- Lu JJ, Chen CH, Bartlett MS, Smith JW, Lee CH. Comparison of six different PCR methods for detection of Pneumocystis carinii. J Clin Microbiol 1995;33:2785-2788.
Treatment Information
For information about treatment please contact CDC-INFO.
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.