Testing for TB Infection
Updated July 11, 2023
The COVID-19 vaccine should not be delayed because of testing for TB infection. TB skin tests and TB blood tests are not expected to affect the safety or the effectiveness of the COVID-19 vaccine. Visit Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States for more information.
There are two types of tests for TB infection: the TB skin test and the TB blood test. A person’s health care provider should choose which TB test to use. Factors in selecting which test to use include the reason for testing, test availability, and cost. Generally, it is not recommended to test a person with both a TB skin test and a TB blood test.
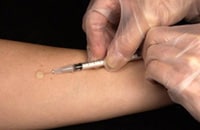
Administering the TB skin test
The TB skin test is also called the Mantoux tuberculin skin test (TST). A TB skin test requires two visits with a health care provider.
On the first visit the test is placed; on the second visit the health care provider reads the test.
- The TB skin test is performed by injecting a small amount of fluid (called tuberculin) into the skin on the lower part of the arm.
- A person given the tuberculin skin test must return within 48 to 72 hours to have a trained health care worker look for a reaction on the arm.
- The result depends on the size of the raised, hard area or swelling.
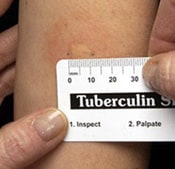
Reading the result of a TB skin test
Positive skin test: This means the person’s body was infected with TB bacteria. Additional tests are needed to determine if the person has latent TB infection or TB disease.
Negative skin test: This means the person’s body did not react to the test, and that latent TB infection or TB disease is not likely.
There is no problem in repeating a TB skin test. If repeated, the additional test should be placed in a different location on the body (e.g., other arm).
The TB skin test is the preferred TB test for children under the age of five.
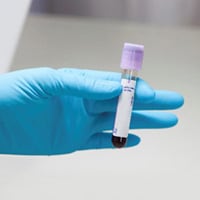
TB blood tests are also called interferon-gamma release assays or IGRAs. Two TB blood tests are approved by the U.S. Food and Drug Administration (FDA) and are available in the United States: the QuantiFERON®-TB Gold Plus (QFT-Plus) and the T-SPOT®.TB test (T-Spot).
A health care provider will draw a patient’s blood and send it to a laboratory for analysis and results.
- Positive TB blood test: This means that the person has been infected with TB bacteria. Additional tests are needed to determine if the person has latent TB infection or TB disease.
- Negative TB blood test: This means that the person’s blood did not react to the test and that latent TB infection or TB disease is not likely.
TB blood tests are the preferred TB test for:
- People who have received the TB vaccine bacille Calmette–Guérin (BCG).
- People who have a difficult time returning for a second appointment to look for a reaction to the TST.
For Patients
For Health Care Providers
- Testing and Diagnosis Guidelines
- National Tuberculosis Controllers Association (NTCA) Testing and Treatment of Latent Tuberculosis Infection in the United States: Clinical Recommendations
Education & Training