
Effectiveness of Incentives for Improving Diabetes-Related Health Indicators in Chronic Disease Lifestyle Modification Programs: a Systematic Review and Meta-Analysis
CME ACTIVITY — Volume 19 — October 27, 2022
LaShonda R. Hulbert, MPH1,2; Shannon L. Michael, PhD, MPH1; Jasmine Charter-Harris, MPH1,3; Charisma Atkins, MPH1; Renée A. Skeete, PhD4; Michael J. Cannon, PhD1 (View author affiliations)
Suggested citation for this article: Hulbert LR, Michael SL, Charter-Harris J, Atkins C, Skeete RA, Cannon MJ. Effectiveness of Incentives for Improving Diabetes-Related Health Indicators in Chronic Disease Lifestyle Modification Programs: a Systematic Review and Meta-Analysis. Prev Chronic Dis 2022;19:220151. DOI: http://dx.doi.org/10.5888/pcd19.220151.
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Preventing Chronic Disease. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.

All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/pcd; (4) view/print certificate.
Release date: October 27, 2022; Expiration date: October 27, 2023
Upon completion of this activity, participants will be able to:
- Distinguish the most common patient incentive applied in randomized trials included in the current meta-analysis
- Assess the effect of patient incentives on body weight and body mass index
- Assess the effect of patient incentives on blood pressure
- Evaluate other cardiometabolic variables that might be improved with patient incentives
EDITOR
Rosemarie Perrin
Editor
Preventing Chronic Disease
Atlanta, Georgia
CME AUTHOR
Charles P. Vega, MD
Health Sciences Clinical Professor of Family Medicine
University of California, Irvine School of Medicine
Charles P. Vega, MD, has the following relevant financial relationships:
Consultant or advisor for: GlaxoSmithKline; Johnson & Johnson Pharmaceutical Research & Development, LLC
AUTHORS
LaShonda Hulbert, MPH
Centers for Disease Control and Prevention
Atlanta, Georgia
CyberData Technologies, Inc.
Herndon, Virginia
Shannon L. Michael, PhD, MPH
Centers for Disease Control and Prevention
Atlanta, Georgia
Jasmine Charter-Harris, MPH
Centers for Disease Control and Prevention
Atlanta, Georgia
Oak Ridge Institute for Science and Education
Oak Ridge, Tennessee
Charisma Atkins, MPH
Centers for Disease Control and Prevention
Atlanta, Georgia
Renée A. Skeete, PhD
Sapodilla Group, LLC
Atlanta, Georgia
Michael J. Cannon, PhD
Centers for Disease Control and Prevention
Atlanta, Georgia
PEER REVIEWED
What is already known on this topic?
Lifestyle modification programs can improve diabetes-related health indicators (eg, body mass index, body weight). Including incentives can make these programs more effective.
What is added by this report?
We demonstrated the effect of incentives in lifestyle modification programs on multiple diabetes-related health indicators and how this effect might vary by incentive domain (ie, incentive type, monetary value, attainment certainty, and incentive schedule).
What are the implications for public health practice?
Using incentives in lifestyle modification programs may improve diabetes-related health indicators, independent of incentive domains. Therefore, programs could exercise flexibility by choosing incentive domains that work for their participants.
Abstract
Introduction
We examined the effectiveness of providing incentives to participants in lifestyle modification programs to improve diabetes-related health indicators: body weight, body mass index (BMI), blood pressure, cholesterol, and hemoglobin A1C (HbA1C). We also examined the potential effect of 4 different incentive domains (ie, type, monetary value, attainment certainty, and schedule) on those indicators.
Methods
We searched Medline, Embase, PsycINFO, and Cochrane Library to identify relevant studies published from January 2008 through August 2021. We used a random-effects model to pool study results and examine between-study heterogeneity by using the I2 statistic and the Cochran Q test. We also conducted moderator analyses by using a mixed-effects model to examine differences between subgroups of incentive domains (eg, incentive type [cash vs other types]).
Results
Our search yielded 10,965 articles, of which 19 randomized controlled trials met our selection criteria. The random-effects model revealed that, relative to the control group, the incentive group had significant reductions in weight (−1.85kg; 95% CI, −2.40 to −1.29; P < .001), BMI (−0.47kg/m2; 95% CI, −0.71 to −0.22; P < .001), and both systolic blood pressure (−2.59 mm HG; 95% CI, −4.98 to −0.20; P = .03) and diastolic blood pressure (−2.62 mm Hg; 95% CI, −4.61 to −0.64; P = .01). A reduction in cholesterol level was noted but was not significant (−2.81 mg/dL; 95% CI, −8.89 to −3.28; P = .37). One study found a significant reduction in hemoglobin A1c (−0.17%; 95% CI, −0.30% to −0.05%; P < .05). The moderator analyses showed that the incentive effect did not vary significantly between the subgroups of the incentive domains, except on weight loss for the attainment certainty domain, suggesting that a variety of incentive subgroups could be equally useful.
Conclusion
Providing incentives in lifestyle modification programs is a promising strategy to decrease weight, BMI, and blood pressure.
Introduction
More than 37 million adults in the US have diabetes, and an additional 96 million have prediabetes, a precursor to type 2 diabetes. Prediabetes is defined as blood glucose levels that are higher than normal, but not high enough to be diagnosed as type 2 diabetes (1). Prevention and management programs are essential for those at risk for and diagnosed with diabetes.
Participating in a lifestyle modification program can help a person develop healthy habits and reduce risks associated with diabetes and related chronic conditions. Results from the National Diabetes Prevention Program have demonstrated that a structured, year-long lifestyle modification program can help participants reduce their risk by coaching them on how to make healthy food choices, reduce stress, and increase physical activity (2,3). Similarly, diabetes self-management education and support services provide essential tools for a person with diabetes to manage the disease and live well (4). However, challenges persist in helping people enroll, stay in, and meet program goals, such as weight loss and blood pressure management (3,5). One promising strategy to address these challenges is the use of incentives (6,7), which can motivate a person to perform a desired action or engage in a behavior (8,9).
Previous systematic reviews and meta-analyses have examined whether incentives can improve health behaviors such as smoking cessation (10,11), getting vaccinations (10), engaging in physical activity (10–16), and improving health indicators such as body weight (11,14). However, some of these reviews did not examine incentives provided solely within the context of a lifestyle modification program (10,11,14,16). Also, several did not examine the effect of different kinds of incentives on program outcomes (10,12,13,15,16).
In contrast, our systematic review and meta-analysis examined a range of diabetes-related health indicators (body weight, body mass index [BMI], blood pressure, cholesterol, and hemoglobin A1C [HbA1c]) and the effect that incentives might have on them in the context of a lifestyle modification program. We also examined the effect by different types of incentives.
Methods
We used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (17) to guide our systematic review and meta-analysis. At each step in the review process, we worked in pairs to review titles and abstracts for inclusion, extract data, and assess the quality of included studies. We resolved any conflicts through discussion among the authors.
Data sources
We searched Medline, Embase, PsycINFO, and Cochrane Library databases for peer-reviewed studies published from January 2008 through August 2021.We chose this date range to identify the most up-to-date and culturally and economically relevant information in close proximity to the time period surrounding the US Congressional mandate for the National Diabetes Prevention Program (https://www.congress.gov/bill/111th-congress/house-bill/4124). The search comprised a combination of key terms related to incentives, lifestyle modification programs, and diabetes-related indicators such as weight, BMI, blood pressure, cholesterol, and HbA1C (Appendix).
Study selection
We selected studies that examined the use of incentives in lifestyle modification programs and their effect on 1 or more diabetes-related health indicators. We considered studies for inclusion if they 1) provided incentives (ie, cash or nonfinancial incentives) to participants; 2) reported on a diabetes-related health indicator(s) (ie, weight, BMI, diastolic and systolic blood pressure, HbA1C, or cholesterol); 3) included adults (≥18 y); 4) occurred in high-income countries (18); 5) included a program that incorporated at least 2 of the following components: nutrition, physical activity, and health education; 6) were published in English in a peer-reviewed journal; and 7) were randomized controlled trials (RCTs).
We excluded studies that focused on programs not designed to modify diabetes-related health indicators (eg, weight, blood pressure); medical interventions for weight loss (eg, gastric bypass); pharmaceutical treatment for weight loss; one-time screenings for preventive services; incentives awarded to health care providers or health systems; or conditions or diseases not of interest (eg, infectious diseases, mental disorders, addictions). We excluded all gray literature, conference and dissertation abstracts, and public health presentations.
Data extraction
We used abstraction forms in DistillerSR (Evidence Partners Incorporated) to screen and manage all articles. Reviewers extracted the following information: study characteristics, study populations, incentive domains, and diabetes-related health indicators. The diabetes-related health indicators of interest were body weight (kilograms [kg] — if only pounds were provided, we converted to kg), body mass index (BMI) (kg/m2), blood pressure (both systolic and diastolic in mm Hg), cholesterol (mg/dL), and HbA1C (%). If multiple publications described the same study by using data from the same participants, we selected the publication with the most complete data and excluded the others.
By using a modified version of a previously published framework (19), we extracted data on 6 incentive domains: 1) Type of incentive — the format that was provided to participants. This included cash, noncash financial (incentives that had a monetary value provided in a form other than currency, such as gift cards), nonfinancial (incentives that did not have a specific monetary value, such as water bottles), and mixed (a combination of 2 or more incentive types). 2) Monetary value — the value or worth of incentives provided. Values were categorized as a high amount, defined by the authors as a value of $270 or more, or a low amount, defined as a value less than $270. This value was chosen because it is the median of the maximum amount of money that participants could earn in 17 of the 19 studies included in our review. 3) Recipient — who received the incentive. Recipients could be individuals, a group of individuals, or mixed (a combination of both). 4) Frequency — how often the incentive was provided. Incentives could be provided either once or multiple times throughout the intervention timeframe. 5) Attainment certainty — how certain it was that a recipient would receive an incentive. This included guaranteed certainty, where incentives were provided regardless of criteria being met; criteria-based guaranteed where participants were required to complete an activity or meet a milestone before the incentive was provided; criteria-based lottery, where participants were required to complete an activity, task, or milestone to become eligible for an incentive lottery; lottery, which was an uncertain chance of receiving an incentive that may have been based on completing an activity or meeting a milestone; and mixed, which was a combination of 2 or more of these strategies. 6) Schedule — how the amount of the incentive was provided to recipients during the study period. Incentive schedules included fixed, where participants received the same incentive amount each time no matter what they did or achieved; variable, where recipients received varying incentive amounts over the intervention period; or mixed, which was a combination of both.
We used The Guide to Community Preventive Services (20) assessment tool to determine the quality (good, fair, or limited) of each study, summarizing across 6 categories: description, sampling, measurement, analysis, interpretation of results, and other.
Statistical analysis
For each study arm (ie, incentive group or control group), we extracted the pre- and post- values for diabetes-related health indicators. Using Comprehensive Meta-Analysis Software Version 3 (21), we calculated the effect sizes as the difference between the mean, pre-to-post change in the incentive group and in the control group. When mean pre-to-post changes were not reported, we used other data provided in the study to calculate the mean difference. For studies that used the same control group to compare with multiple incentive groups, we used the mean of the incentive groups in the analyses. A negative difference in means signified that the incentive group lost more weight, had a greater decrease in BMI, or had a greater decrease in blood pressure than the control group.
We used forest plots to compare results of the studies, including differences of means, 95% CIs, and P values. We used random effects models, which consider between-study variations, to calculate pooled effect-size estimates. We evaluated the overall effect of studies using z statistics with P < .05 considered significant. We assessed the risk for publication bias by visually inspecting funnel plots and assessing the degree of asymmetry in the distribution of effect sizes by using the techniques of Begg and Mazumdar (22) and Egger et al (23). When publication bias was detected, we used trim-and-fill procedures to correct for the possibility of missing studies (21).
We assessed heterogeneity by 95% CIs, I-squared (I2) values, Q statistics, and their associated P values. We interpreted the I2 value, per the Cochrane Handbook (24) as follows: unimportant heterogeneity (0%–40%), moderate heterogeneity (30%–60%), substantial heterogeneity (50%–90%), and considerable heterogeneity (75%–100%). We further assessed heterogeneity by conducting sensitivity analyses that removed individual studies that were potential outliers and assessed the updated findings (ie, forest plots, I2 values, and Q statistics). We also examined differences between study quality and types of lifestyle modification programs provided to the incentive and control groups to see how they affected heterogeneity and overall effect size.
To assess the impact of different incentive domains, we conducted moderator analyses with categorical variables by using mixed-effects models. We assessed the effect on body weight and BMI for subgroups within 4 incentive domains: including 1) type (cash vs other types); 2) monetary value (high vs low — we defined high as a value of $270 or more, which is the median of the amount of money that participants could earn in the 17 studies providing financial incentives); 3) attainment certainty (criteria-based guaranteed vs other), and 4) schedule (fixed vs other). Because at least 2 studies in each subgroup are needed to conduct the analysis, we were only able to conduct the moderator analyses for body weight and BMI and for 4 of the 6 incentive domains.
Results
Our initial searches returned 10,965 articles (Figure 1). After removing duplicates, we screened 8,240 study titles and abstracts for possible inclusion. On the basis of the inclusion/exclusion criteria, we selected 95 studies for full-text review and identified 19 studies that met the inclusion criteria.
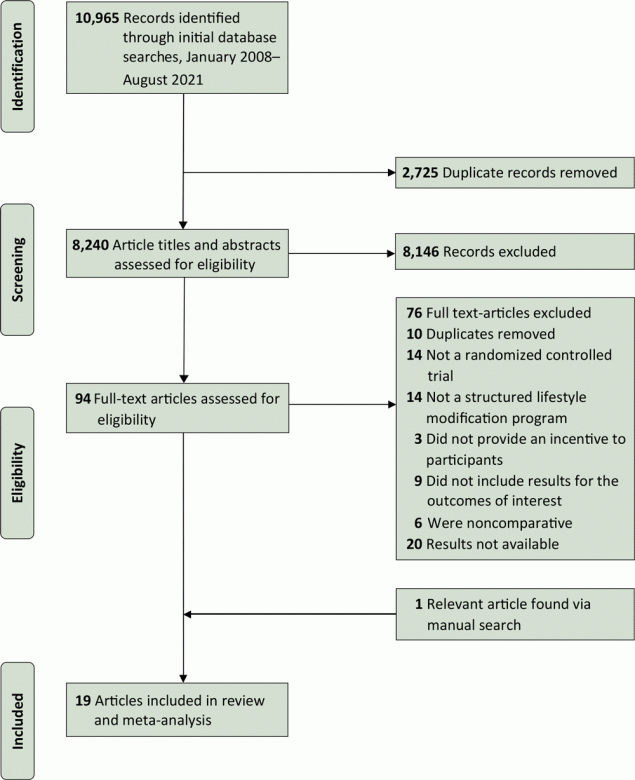
![]()
Figure 1.
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the study selection process and literature search from 4 databases: Medline, Embase, PsycINFO, and Cochrane Library, from January 2008 through August 2021. We chose this date range to identify the most up-to-date and culturally and economically relevant information in close proximity to the time period surrounding the US Congressional mandate for the National Diabetes Prevention Program (https://www.congress.gov/bill/111th-congress/house-bill/4124). [A text version of the figure is available.]
Study characteristics
Of the 19 studies included in our review, 14 were conducted in the United States, 2 in Australia, 1 in Singapore, 1 in Scotland, and 1 in South Korea (Table 1). Most of the studies had fewer than 500 participants, with a total of 5,291 participants across all studies. The participant age range across studies was 18 to 80 years.
The most frequently reported setting of the included studies was internet-based programs (n = 6) (26,29,35,37,42,43). The time frame for the lifestyle modification programs was from 12 weeks to 24 months. All included studies measured weight, 7 measured BMI (25–28,31,32,38), 4 measured blood pressure (25,27,32,38), 2 measured cholesterol (26,38), and 1 measured hemoglobin A1c (HbA1c) (28). The quality scores of the studies varied: 4 good (35,39,41,43); 13 fair (25–27,29–34,37,38,40,42); and 2 (28,36) limited.
Incentive domains
Among the 19 RCTs, the most common incentive type was cash (Table 2). Most studies reported a monetary value greater than or equal to the median of $270 (n = 9) (30–34,37,38,40,43), and most incentives were distributed to individual recipients (n = 15) (26–29,31–33,35–40,42,43). Incentives were frequently provided multiple times over the course of the included programs (n = 16) (25–30,33,34,36–43); most studies used a criteria-based guaranteed approach (n = 15) (25,26,29–34,37–43), and 13 studies (26,27,29–31,33–38,41,42) distributed the incentive to participants on a variable schedule.
Magnitude and direction of effects
We found a significant overall mean effect for providing incentives in lifestyle modification programs for 4 diabetes-related health indicators: body weight, BMI, and both diastolic and systolic blood pressure (Figure 2).
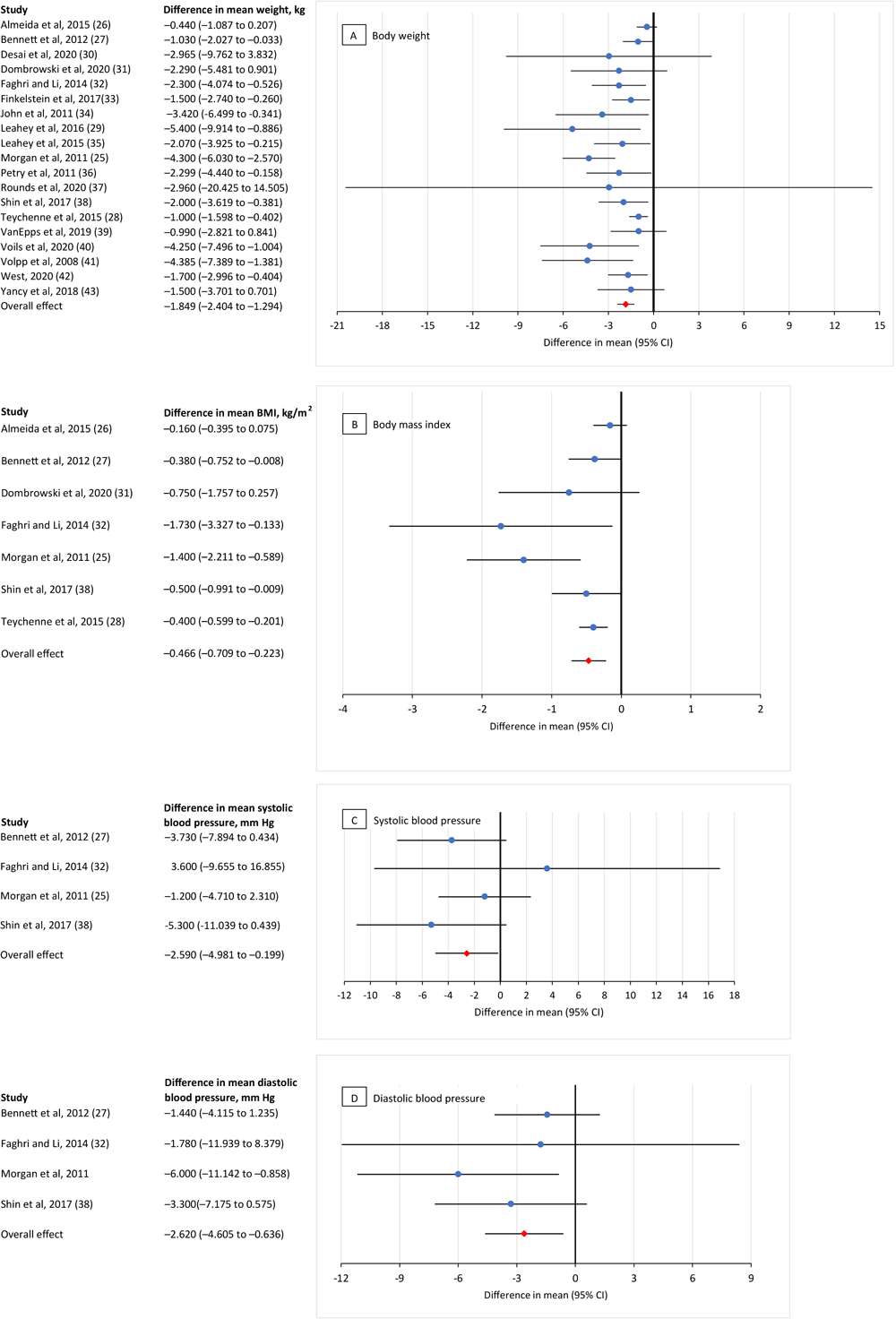
![]()
Figure 2.
Meta-analysis of the effects of incentives (ie, cash or nonfinancial incentives) on improving diabetes-related health indicators in chronic disease lifestyle modification programs. A, the effect of incentives on body weight (kg); calculations were based on 23 comparisons reported in 19 studies (25–43). B, the effect of incentives on body mass index (kg/m2
); calculations were based on 7 comparisons reported in 7 studies (25–28,31,32,38). C, the effect of incentives on systolic blood pressure (mm Hg); calculations were based on 4 comparisons reported in 4 studies (25,27,32,38). D, the effect of incentives on diastolic blood pressure (mm Hg); calculations were based on 4 comparisons reported in 4 studies (25,27,32,38). Values <0 indicate an incentive effect, and values >0 indicate no incentive effect. [A tabular version of this figure is available.]
Weight. The pooled mean difference for weight was −1.85 kg (95% CI, −2.40 to −1.29; Z = −6.53, P < .001), indicating that the incentive group lost more weight (−1.85 kg or 4.1lb) than the control group.
BMI. The pooled mean difference for BMI was −0.47 kg/m2 (95% CI, −0.71 to −0.22; Z = −3.76, P < .001), meaning the incentive group decreased their BMI by 0.47 kg/m2 more than the control group.
Blood pressure. The pooled mean difference for systolic blood pressure was −2.59 mm Hg (95% CI, −4.98 to −0.20; Z = −2.12, P = .03), and for diastolic blood pressure it was −2.62 mm Hg (95% CI, −4.61 to −0.64; Z = −2.59, P = .01) meaning that the incentive group had a greater decrease in their systolic and diastolic blood pressure than the control group.
Cholesterol and HbA1C. Two studies (26,38) examined total cholesterol as an outcome measure and both were nonsignificant, with a pooled mean difference of −2.81 mg/dL (95% CI, −8.89 to −3.28; Z = −0.91, P = .37). We did not have at least 2 studies to conduct a meta-analysis for HbA1C; in the only RCT (28) examining that value, the incentive group had a significantly greater decrease in their HbA1C levels than the control group (mean difference, −0.17%; 95% CI, −0.30 to −0.05, P = <.05).
Publication bias
The funnel plots of the effect sizes for weight and BMI were asymmetrical, suggesting possible publication bias for studies with nonsignificant effects. This was supported by the significant findings from the Begg and Mazumdar (22) publication bias test results for weight (Kendall’s tau b = −0.33, with a one-tailed P value of .03) and BMI (Kendall’s tau b = −0.57, with a one-tailed P value of .04). In addition, the Egger et al (23) publication bias test showed an intercept of −1.78 with a one-tailed P value of <.001 for weight, and an intercept of −1.93 with a one-tailed P value of .02 for BMI. We used Duval and Tweedie’s Trim and Fill (21) analysis to understand this further and found that 9 potential studies might be missing from the weight meta-analysis because of publication bias. With these additional studies, the estimated effect size for weight would be smaller, −1.20 kg, but still significant (95% CI, −1.80 to −0.60). For BMI, we found that 3 potential studies might be missing from the BMI meta-analysis, and with these additional studies, the estimated effect size for BMI would be smaller, −0.34 kg/m2, but still significant (95% CI, −0.62 to −0.06).
The funnel plots of the effect sizes were symmetrical for diastolic and systolic blood pressure, and the trim-and-fill analysis suggested no adjustment to the mean effect size, indicating no evidence of publication bias.
Heterogeneity and subgroup analyses
We found moderate to substantial heterogeneity for the effect size of incentives on weight (I2 = 51%; Q = 36.43; P < .01) and BMI (I2 = 54%; Q = 12.95; P = .04), so we conducted subgroup analyses to better understand the potential sources of heterogeneity.
First, we identified potential study outliers as those with standard residuals ≥1.96. One study was identified as an outlier for weight and BMI (25). When we removed this study as a potential outlier, the I2 percentage decreased to nonsignificant heterogeneity (I2 = 31%, Q = 24.77, P = .10) for weight and for BMI (I2 = 24%, Q = 6.60, P = .25). We chose to keep this study in both meta-analyses because it did not change the significance and only slightly lowered the pooled effect sizes (weight: from −1.85 kg to −1.54 kg; BMI: from −0.47 kg/m2 to −0.36 kg/m2).
Second, we assessed heterogeneity as a function of study quality but did not find a consistent pattern. We found that for weight, studies rated as fair (25–27,29,30–34,37,38,40,42) had a stronger effect size (−2.07 kg) and moderate to substantial heterogeneity (I2 = 60%) compared with studies rated as good (35,39,41,43) (−1.92 kg; I2 = 20%, unimportant heterogeneity) and limited (28,36) (−1.23 kg; I2 = 24%, unimportant heterogeneity). For BMI, the assessment was constrained because 6 studies were rated as fair (25–27,31,32,38) but only one was rated limited (28). The fair studies had a stronger effect size (−0.56 kg/m2) and moderate to substantial heterogeneity (I2 = 61%) compared with the study rated as limited (−0.40kg/m2; I2 = 0%, unimportant heterogeneity).
Lastly, we assessed heterogeneity as a function of program characteristics. For weight, there were 5 studies that provided slightly different lifestyle modification programs to the incentive group and the control group (25–29), whereas 14 studies provided the same program to both groups, with the incentive being the only difference (30–43). We found that the effect size was greater for the 14 studies that provided the same program to the incentive and control groups (−1.99 kg) compared with the 5 studies that did not (−1.62 kg). In addition, for those 14 studies the I2 was 0% and nonsignificant (Q = 7.59; P = .87), which means that the differences across those studies are due to sampling error, not differences in true effect sizes. For the 5 studies that did not provide the same program, the I2 was 80% and significant (Q = 20.51; P = <.001), which means the amount of variability across the studies cannot be explained by chance alone. We found similar results for BMI, where the three studies (31,32,38) that provided the same program to the incentive group and control group had a greater effect size (−0.65 kg/m2) and unimportant heterogeneity (I2 = 6%; Q = 2.15; P = .34), compared with the lower effect size (−0.41 kg/m2) with substantial heterogeneity (I2 = 67%; Q = 9.20; P = .03) for the 4 studies (25–28) that provided a slightly different program to both groups.
We found heterogeneity to be unimportant for diastolic (I2 = 0%, Q = 2.55; P = .47) and systolic (I2 = 0%, Q = 2.58, P = .46) blood pressure, so we did not conduct any further analyses.
Moderator analyses for incentive domains
The effect sizes between most of the subgroups of the incentive domains were not significantly different (Table 3), indicating no difference between each subgroup’s ability to lower body weight and BMI. For the attainment certainty domain, both subgroups had an effect on weight, but the difference between them was significant, indicating that the criteria-based guaranteed subgroup might have a greater effect on weight (−2.20 kg) than other attainment criteria (−1.15 kg).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to examine the effect of incentives on multiple diabetes-related health indicators in the context of a lifestyle modification program. Our findings suggest that incentives are an effective strategy to lower body weight, BMI, and systolic and diastolic blood pressure. Of note, the results for HbA1c were significant, but only 1 study reported on this (28). Although the results for cholesterol were promising, they were not significant. The results of the subgroup analyses showed that in programs where the only difference between incentive and control groups was the incentive, a larger effect on body weight and BMI was observed, which is indicative of the usefulness of incentives and the reliability of the effect size.
The results of our meta-analysis showed a nearly 2 kg greater weight loss and a significant reduction in BMI when incentives were provided. This finding has important health implications considering that a large study reported a 16% reduction in diabetes risk for every kg of weight lost (44), and others have shown that weight loss in conjunction with a lifestyle modification program can lower the risk for cardiovascular disease (45–47). Additionally, for those with type 2 diabetes who are overweight or have obesity, there are significant benefits for reducing not only body weight, but blood pressure, and cholesterol as well (45).
Providing incentives to participants in lifestyle modification programs was shown to be effective for reducing systolic and diastolic blood pressure. These findings could be beneficial for programs that seek to help participants achieve the ideal blood pressure (ie, <140/90 mm Hg), especially those programs associated with the prevention and management of chronic diseases such as type 2 diabetes (48). Reducing blood pressure protects against cardiovascular events for people at risk for and diagnosed with diabetes and is critical to managing the disease (48,49).
The results of the moderator analyses showed that nearly all the subgroups in each incentive domain (eg, high vs low monetary value) had a significant effect on reducing body weight and BMI. Some of the effect sizes were larger than others, suggesting that certain incentive domains might be more effective. For example, for the incentive type domain, the cash subgroup showed a −1.79 kg (P = <.001) decrease in weight, whereas the other subgroup types showed a −2.03 kg (P = <.001) decrease. However, the differences in effect sizes across most subgroups were mostly nonsignificant. Therefore, it seems reasonable for lifestyle modification programs to use a variety of incentive domain subgroups.
Future studies could consider the effectiveness of various types of incentives for specific populations, cultures, certain health indicators like cholesterol and HbA1c, and certain settings to determine whether incentives could reduce disparities in the outcomes of lifestyle modification programs (50,51). Although recipients of incentives generally find them acceptable (52–55), other individual and programmatic considerations, such as demographic characteristics or funding sources, deserve further exploration (53). Similarly, researchers could consider using a common framework (10–12,19) for reporting incentives in addition to reporting program and participant characteristics so that they might be examined simultaneously. Also, evaluating incentives for cost-effectiveness could ensure that resources spent on them make economic sense for programs. Future research might also seek to better understand how using incentives might affect intrinsic motivation or habit formation, especially because lifestyle modification requires a long-term commitment to the habitual behaviors that reduce risks (56–58). One study has reported that it is unlikely that incentives undermine intrinsic motivation and could possibly enhance intrinsic motivation to participate in a program (59).
Limitations
Our study has several limitations. Variability across lifestyle modification programs and incentive domains likely contributed to the heterogeneity of our results. Nevertheless, we found no significant differences in effect sizes according to the subgroup analysis for heterogeneity. Also, 2 of the incentive subgroup moderator analyses consisted of 2 studies, so we interpreted those results with caution. Finally, we were not able to include other factors that could influence participation or success, such as race or ethnicity of participants or the coach, the socioeconomic status of participants, or other incentive domains, such as incentive timing (19), so our results are limited to what we could abstract from the existing studies.
Strengths
Our study has several strengths. We had enough RCT studies to include in a meta-analysis with multiple diabetes-related health indicators, and most of them were considered of good or fair quality, mostly on the basis of limitations of the sample designs and the interpretation of study results. We used subgroup analyses to address heterogeneity, used moderator analyses to understand the effect of several incentive domains on health indicators, and adjusted for potential publication bias. Through these analyses we determined that the observed effect of the incentives could not be attributed to outlier studies or publication bias and that the effect sizes were consistent across incentive domains. Furthermore, for the programs that differed only by the incentive, we observed a strong effect and low heterogeneity in these studies for body weight and BMI. These findings provide more confidence that the use of incentives in lifestyle modification programs for chronic disease can lead to better outcomes.
Conclusion
Using incentives in lifestyle modification programs is a promising strategy for adults at risk for or diagnosed with type 2 diabetes to reduce body weight, BMI, blood pressure, and, potentially, HbA1c. Because our analysis showed effectiveness of incentives on multiple health indicators, we were able to add to the literature regarding the use of incentives to promote lifestyle modification. Chronic disease prevention and management programs can consider incentives as a tool to increase participant success.
Acknowledgments
The authors have no conflict of interest to declare and no sources of funding that could have influenced the outcome of this work. No copyrighted materials were used in this article. The findings and conclusions reported in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors acknowledge Akimi Smith, MPH, Sarah Dorvil, MPH, Joanna Taliano, MA, MLS, and Robin Soler, PhD, for their contributions to this work.
Author Information
Corresponding Author: LaShonda R. Hulbert, MPH, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 4770 Buford Highway NE, Mailstop 107-3, Atlanta, GA 30341. Telephone: (404) 498-5671. Email: NYT2@cdc.gov.
Author Affiliations: 1Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia. 2CyberData Technologies, Inc, Herndon, Virginia. 3Oak Ridge Institute for Science and Education, Oak Ridge, Tennessee. 4Sapodilla Group, LLC, Atlanta, Georgia.
References
- Centers for Disease Control and Prevention. National diabetes statistics report 2022. Accessed January 20, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med 2013;44(4 Suppl 4):S346–51. CrossRef PubMed
- Ely EK, Gruss SM, Luman ET, Gregg EW, Ali MK, Nhim K, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40(10):1331–41. CrossRef PubMed
- Boren SA, Fitzner KA, Panhalkar PS, Specker JE. Costs and benefits associated with diabetes education: a review of the literature. Diabetes Educ 2009;35(1):72–96. CrossRef PubMed
- Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020. Accessed February 28, 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Nhim K, Gruss SM, Porterfield DS, Jacobs S, Elkins W, Luman ET, et al. Using a RE-AIM framework to identify promising practices in National Diabetes Prevention Program implementation. Implement Sci 2019;14(1):81. CrossRef PubMed
- Jeffery RW. Financial incentives and weight control. Prev Med 2012;55(Suppl):S61–7. CrossRef PubMed
- Haff N, Patel MS, Lim R, Zhu J, Troxel AB, Asch DA, et al. The role of behavioral economic incentive design and demographic characteristics in financial incentive-based approaches to changing health behaviors: a meta-analysis. Am J Health Promot 2015;29(5):314–23. CrossRef PubMed
- Higgins ST, Silverman K, Sigmon SC, Naito NA. Incentives and health: an introduction. Prev Med 2012;55(Suppl):S2–6. CrossRef PubMed
- Giles EL, Robalino S, McColl E, Sniehotta FF, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta-analysis. PLoS One 2014;9(3):e90347. CrossRef PubMed
- Mantzari E, Vogt F, Shemilt I, Wei Y, Higgins JP, Marteau TM. Personal financial incentives for changing habitual health-related behaviors: a systematic review and meta-analysis. Prev Med 2015;75:75–85. CrossRef PubMed
- Mitchell MS, Goodman JM, Alter DA, John LK, Oh PI, Pakosh MT, et al. Financial incentives for exercise adherence in adults: systematic review and meta-analysis. Am J Prev Med 2013;45(5):658–67. CrossRef PubMed
- Mitchell MS, Orstad SL, Biswas A, Oh PI, Jay M, Pakosh MT, et al. Financial incentives for physical activity in adults: systematic review and meta-analysis. Br J Sports Med 2020;54(21):1259–68. CrossRef PubMed
- Barte JCM, Wendel-Vos GCW. A systematic review of financial incentives for physical activity: the effects on physical activity and related outcomes. Behav Med 2017;43(2):79–90. CrossRef PubMed
- Ananthapavan J, Peterson A, Sacks G. Paying people to lose weight: the effectiveness of financial incentives provided by health insurers for the prevention and management of overweight and obesity – a systematic review. Obes Rev 2018;19(5):605–13. CrossRef PubMed
- Gong Y, Trentadue TP, Shrestha S, Losina E, Collins JE. Financial incentives for objectively-measured physical activity or weight loss in adults with chronic health conditions: A meta-analysis. PLoS One 2018;13(9):e0203939. CrossRef PubMed
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10(1):89. CrossRef PubMed
- The World Bank Group. High income. Accessed February 28, 2020. 2022. https://data.worldbank.org/country/XD
- Adams J, Giles EL, McColl E, Sniehotta FF. Carrots, sticks and health behaviours: a framework for documenting the complexity of financial incentive interventions to change health behaviours. Health Psychol Rev 2014;8(3):286–95. CrossRef PubMed
- Guide to Community Preventive Services. Methods manual for Community Guide systematic reviews. 2021.Accessed December 15, 2021. https://www.thecommunityguide.org/methods-manual/effectiveness-review-methods
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis version 3. Biostat 2013. Accessed January 31, 2021. https://www.meta-analysis.com/downloads/Meta-Analysis%20Manual%20V3.pdf.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088–101. CrossRef PubMed
- Egger M, Davey Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. CrossRef PubMed
- Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta‐analyses. Cochrane handbook for systematic reviews of interventions version 6.3 2022. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). John Wiley and Sons; 2019, pp. 241–84. Accessed January 21, 2021. www.training.cochrane.org/handbook
- Morgan PJ, Collins CE, Plotnikoff RC, Cook AT, Berthon B, Mitchell S, et al. Efficacy of a workplace-based weight loss program for overweight male shift workers: the Workplace POWER (Preventing Obesity Without Eating like a Rabbit) randomized controlled trial. Prev Med 2011;52(5):317–25. CrossRef PubMed
- Almeida FA, You W, Harden SM, Blackman KC, Davy BM, Glasgow RE, et al. Effectiveness of a worksite-based weight loss randomized controlled trial: the worksite study. Obesity (Silver Spring) 2015;23(4):737–45. CrossRef PubMed
- Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, et al. ; Be Fit, Be Well Study Investigators. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med 2012;172(7):565–74. CrossRef PubMed
- Teychenne M, Ball K, Salmon J, Daly RM, Crawford DA, Sethi P, et al. Adoption and maintenance of gym-based strength training in the community setting in adults with excess weight or type 2 diabetes: a randomized controlled trial. Int J Behav Nutr Phys Act 2015;12(1):105. CrossRef PubMed
- Leahey TM, Fava JL, Seiden A, Fernandes D, Doyle C, Kent K, et al. A randomized controlled trial testing an Internet delivered cost-benefit approach to weight loss maintenance. Prev Med 2016;92:51–7. CrossRef PubMed
- Desai JR, Vazquez-Benitez G, Taylor G, Johnson S, Anderson J, Garrett JE, et al. The effects of financial incentives on diabetes prevention program attendance and weight loss among low-income patients: the We Can Prevent Diabetes cluster-randomized controlled trial. BMC Public Health 2020;20(1):1587. CrossRef PubMed
- Dombrowski SU, McDonald M, van der Pol M, Grindle M, Avenell A, Carroll P, et al. Game of Stones: feasibility randomised controlled trial of how to engage men with obesity in text message and incentive interventions for weight loss. BMJ Open 2020;10(2):e032653. CrossRef PubMed
- Faghri PD, Li R. Effectiveness of financial incentives in a worksite diabetes prevention program. Open Obes J 2014;6(1):1–12. CrossRef PubMed
- Finkelstein EA, Tham KW, Haaland BA, Sahasranaman A. Applying economic incentives to increase effectiveness of an outpatient weight loss program (TRIO) — a randomized controlled trial. Soc Sci Med 2017;185:63–70. CrossRef PubMed
- John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med 2011;26(6):621–6. CrossRef PubMed
- Leahey TM, Subak LL, Fava J, Schembri M, Thomas G, Xu X, et al. Benefits of adding small financial incentives or optional group meetings to a web-based statewide obesity initiative. Obesity (Silver Spring) 2015;23(1):70–6. CrossRef PubMed
- Petry NM, Barry D, Pescatello L, White WB. A low-cost reinforcement procedure improves short-term weight loss outcomes. Am J Med 2011;124(11):1082–5. CrossRef PubMed
- Rounds T, Crane M, Harvey J. The impact of incentives on weight control in men: a randomized controlled trial. Am J Men Health 2020;14(1):1557988319895147. CrossRef PubMed
- Shin DW, Yun JM, Shin JH, Kwon H, Min HY, Joh HK, et al. Enhancing physical activity and reducing obesity through smartcare and financial incentives: a pilot randomized trial. Obesity (Silver Spring) 2017;25(2):302–10. CrossRef PubMed
- VanEpps EM, Troxel AB, Villamil E, Saulsgiver KA, Zhu J, Chin JY, et al. Effect of process- and outcome-based financial incentives on weight loss among prediabetic New York Medicaid patients: a randomized clinical trial. Am J Health Promot 2019;33(3):372–80. CrossRef PubMed
- Voils CI, Pendergast J, Hale SL, Gierisch JM, Strawbridge EM, Levine E, et al. A randomized feasibility pilot trial of a financial incentives intervention for dietary self-monitoring and weight loss in adults with obesity. Transl Behav Med 2020;11(4):954–69. PubMed
- Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA 2008;300(22):2631–7. CrossRef PubMed
- West DS, Krukowski RA, Finkelstein EA, Stansbury ML, Ogden DE, Monroe CM, et al. Adding financial incentives to online group-based behavioral weight control: an RCT. Am J Prev Med 2020;59(2):237–46. CrossRef PubMed
- Yancy WS Jr, Shaw PA, Wesby L, Hilbert V, Yang L, Zhu J, et al. Financial incentive strategies for maintenance of weight loss: results from an internet-based randomized controlled trial. Nutr Diabetes 2018;8(1):33. CrossRef PubMed
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29(9):2102–7. CrossRef PubMed
- American Diabetes Association. 8. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes–2021. Diabetes Care 2021;44(Suppl 1):S100–10. CrossRef PubMed
- Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med 2015;163(6):437–51. CrossRef PubMed
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. ; Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374(9702):1677–86. CrossRef PubMed
- American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes–10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes–2020. Diabetes Care 2020;43(Suppl 1):S111–34. CrossRef PubMed
- Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens 2011;29(7):1253–69. CrossRef PubMed
- Cannon MJ, Masalovich S, Ng BP, Soler RE, Jabrah R, Ely EK, et al. Retention among participants in the National Diabetes Prevention Program Lifestyle Change Program, 2012-2017. Diabetes Care 2020;43(9):2042–9. CrossRef PubMed
- Jackson MC, Dai S, Skeete RA, Owens-Gary M, Cannon MJ, Smith BD, et al. An examination of gender differences in the National Diabetes Prevention Program’s Lifestyle Change Program. Diabetes Educ 2020;46(6):580–6. CrossRef PubMed
- Shea JA, Adejare A, Volpp KG, Troxel AB, Finnerty D, Hoffer K, et al. Patients’ views of a behavioral intervention including financial incentives. Am J Manag Care 2017;23(6):366–71. PubMed
- Bigsby E, Seitz HH, Halpern SD, Volpp K, Cappella JN. Estimating acceptability of financial health incentives. Health Educ Behav 2017;44(4):513–8. CrossRef PubMed
- Blondon KS. Patient attitudes about financial incentives for diabetes self-management: A survey. World J Diabetes 2015;6(5):752–8. CrossRef PubMed
- Blondon K, Klasnja P, Coleman K, Pratt W. An exploration of attitudes toward the use of patient incentives to support diabetes self-management. Psychol Health 2014;29(5):552–63. CrossRef PubMed
- Curry SJ, Wagner EH, Grothaus LC. Evaluation of intrinsic and extrinsic motivation interventions with a self-help smoking cessation program. J Consult Clin Psychol 1991;59(2):318–24. CrossRef PubMed
- Mogler BK, Shu SB, Fox CR, Goldstein NJ, Victor RG, Escarce JJ, et al. Using insights from behavioral economics and social psychology to help patients manage chronic diseases. J Gen Intern Med 2013;28(5):711–8. CrossRef PubMed
- Thirumurthy H, Asch DA, Volpp KG. The uncertain effect of financial incentives to improve health behaviors. JAMA 2019;321(15):1451–2. CrossRef PubMed
- Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? Comparing behaviors studied in psychological and economic literatures. Health Psychol 2013;32(9):950–7. CrossRef PubMed
Tables
| First author, year, country | Settinga | Populationb | Number incentive group/nonincentive group | Program description | Intervention duration, final measurement periodc | Program health indicators of interest | Study quality ratingsd |
|---|---|---|---|---|---|---|---|
| Almeida et al, 2015, US (26) | Internet-based, worksite programe | Participants aged ≥18 years, BMI ≥25 kg/m2, not currently pregnant, free of serious medical conditions (eg, recent heart attack), access to internet | 789/1,001 | Intervention group received nutrition and physical activity incentives for weight loss and incentives. Comparison group received educational materials focused on nutrition and physical activity without incentives | 12 Months; final measurement at month 6 | Weight, BMI, cholesterol | Fair |
| Bennett et al, 2012, US (27) | Community health centers | Participants aged ≥21 years, BMI of 30–50 kg/m2, weight <180 kg, and diagnosed hypertension | 180/185 | Intervention group participated in weight loss activities, engaged with CHWs, and received incentives; comparison group received a self-help booklet and no incentives | 24 months, final measurement at month 24 | Weight, BMI, systolic and diastolic blood pressure | Fair |
| Desai et al, 2020, US (30) | Primary care clinics | Participants aged 18–74 years at risk for type 2 diabetes and enrolled in Medicaid | 568/279 | Intervention group received the 12-month group-delivered DPP, based on the CDC National DPP and incentives. Comparison group received no incentives | 12 months, final measurement at month 12 | Weight | Fair |
| Dombrowski et al, 2020, Scotland (31) | Two public health care regionsf | Men aged ≥18 years, BMI ≥30 kg/m2 and/or waist circumference of ≥40 inches, access to a cellular telephone | 36/33g | Intervention group received narrative text message engagement, physical activity and nutrition components, and incentives. Comparison group received no incentives | 12 months, final measurement at month 12 | Weight, BMI | Fair |
| Faghri and Li, 2014, US (32) | Worksite, long-term nursing home facilities | Employees of long-term care facility overweight or with obesity, and at risk for type 2 diabetes | 35/38h | Intervention group received physical activity and nutrition components and incentives. Comparison group received no incentives | 16 weeks, final measurement at week 16 | Weight, BMI, systolic and diastolic BP | Fair |
| Finkelstein et al, 2017, Singapore (33) | Fitness center at Singapore General Hospital | Participants aged 21–65 years; BMI of 25–40 kg/m2 | 107/54 | Intervention group received goal setting and tracking, diet and nutrition management, physical activity resources, and incentives. Comparison group received no incentives | 8 months, final measurement at month 8 | Weight | Fair |
| John et al, 2011, US (34) | Veterans Affairs Medical Center | Veterans aged 30–70 years, BMI of 30–40 kg/m2 | A:22, B:22/ control:22 | Intervention groups received a weight monitoring program (dietary counseling and weight loss), and 1of 2 incentive plans. Comparison group received no incentives | 32 weeks, final measurement at week 32 | Weight | Fair |
| Leahey et al, 2015, US (35) | Internet-based programe | Participants aged 18–70 years, BMI ≥25 kg/m2 | 89/91 | Intervention group received nutrition, physical activity, weight tracking resources, and incentives. Comparison group received no incentives | 3 months, final measurement at month 3 | Weight | Good |
| Leahey et al, 2016, US (29) | Internet-based programe | Participants aged 18–70 years, BMI ≥25 kg/m2 |
A:25, B:26/ control:24 | Intervention group received coaching, a web-based weight maintenance program based on National DPP, and incentives with either a professional coach or peer coach. Comparison group received a single 1-hour group session and no incentives | 12 months, final measurement at month 12 | Weight | Fair |
| Morgan et al, 2011, Australia (25) | Worksite wellness program | Male shift workers at an aluminum company aged 18–65 years who were overweight or had obesity | 65/45 | Intervention group received a group session on nutrition and physical activity, self-monitoring, goal setting, and incentives. Comparison group was placed on a wait list and did not receive incentives | 12 weeks, final measurement at week 14 | Weight, BMI, systolic and diastolic blood pressure | Fair |
| Petry et al, 2011, US (36) | Setting not specified | Participants aged 18–65, BMI of 30–39.9 kg/m2, and blood pressure of 110/70–140/90 mm Hg | 28/28 | Intervention group received nutrition and physical activity components through supportive lifestyle counseling and incentives. Comparison group received no incentives | 12 weeks, final measurement at week 12 | Weight | Limited |
| Rounds et al, 2020, US (37) | Internet-based programe | Men aged 18–65 years and a BMI of 25–40 kg/m2 | 34/24 | Intervention group received nutrition and physical activity components, online lessons, and incentives. Comparison group received no incentives | 12 weeks, final measurement at week 24 | Weight | Fair |
| Shin et al, 2017, South Korea (38) | Academic institution | Male students aged 19–45 years, BMI ≥27 kg/m2, access to smartphone | 35/35i | Intervention groups received an individualized education session on nutrition and physical activity and incentives. Comparison group received no incentives | 12 weeks, final measurement at week 12 | Weight, BMI, systolic and diastolic BP, cholesterol | Fair |
| Teychenne et al, 2015, Australia (28) | Health and fitness centers or home | Participants aged 40–75 years with type 2 diabetes or BMI ≥25 kg/m2 | 162/156 | Intervention group received supervised group exercise sessions, behavioral counseling, newsletters, and incentives. Comparison group received the supervised group exercise sessions and no incentives | 12 months, final measurement at month 12 | Weight, BMI, HbA1c | Limited |
| VanEpps et al, 2019, US (39) | Community health center, Medicaid managed care plan center, or a local YMCA | Participants aged 18- 64 years, and at risk for type 2 diabetes | 170/170j | Intervention group received group sessions with physical activity and nutrition components based on the National DPP, and incentives. Comparison group received no incentives | 16 weeks, final measurement at week 16 | Weight | Good |
| Voils et al, 2020, US (40) | University medical center | Participants aged 18–70 years, BMI ≥30 kg/m2, access to a cellular telephone with a data plan | 23/24k | Intervention group received the standard behavioral weight loss sessions, nutrition and physical activity components, motivational text messages, and incentives. Comparison group received the standard behavioral weight loss sessions and no incentives | 24 weeks, final measurement at week 24 | Weight | Fair |
| Volpp et al, 2008, US (41) | Veterans Affairs medical center | Participants aged 30–70 years, BMI of 30–40 kg/m2 | A:19, B:19/ control:19 | Intervention group received an individual session with a dietician, physical activity, nutrition components, and 1 of 2 incentive plans. Comparison group received no incentives | 16 weeks, final measurement at week 16 | Weight | Good |
| West et al, 2020, US (42) | Internet-based programe | Participants aged ≥18 years, BMI of 25–50 kg/m2, and access to the internet |
206/212 | Intervention group received online group-based behavioral weight control sessions based on the National DPP, with physical activity and nutrition components and incentives. Comparison group received no incentives | 6 months, final measurement at month 6 | Weight | Fair |
| Yancy et al, 2018, US (43) | Internet-based programe | Participants enrolled in Weight Watchers aged 30–80 years, BMI of 30–45 kg/m2 | A:75, B:77/ control: 39 | Intervention group received physical activity and nutrition components, text message engagement, and was assigned to 1 of 2 incentive plans. Comparison group received no incentives | 6 months, final measurement at month 6 | Weight | Good |
Abbreviations: BP, blood pressure; BMI, body mass index; CDC, Centers for Disease Control and Prevention, CHW, community health worker; National DPP, National Diabetes Prevention Program; YMCA, Young Men’s Christian Association.
a The location where the study took place or the location of the principal investigators where participants would report.
b Because age, BMI, and gender (if focused only on males or females) were consistently reported in the included studies, we were able to include this demographic information across all studies for the populations of focus. Other demographic information such as race, was not consistently reported across the studies and we therefore did not include it here.
c The length of time that participants receive program support. We defined final measurement period as the last point at which health indicators were measured and the incentive groups were still receiving support — this is the health indicator measure used for our meta-analysis.
d Consists of 3 categories — good, fair, and limited — measuring across 6 categories: description, sampling, measurement, analysis, interpretation of results, and other. These ratings are explained in the Guide to Community Preventive Services assessment tool (15).
e Defined as a lifestyle modification program that is delivered via the internet, including access to program materials and engagement with program facilitators and peers if applicable.
f Scotland is divided into health care regions in which the public receives health care services.
g This study had 3 arms. For the purposes of our analysis, we compared the arm that received the intervention plus incentives to the arm that received only the intervention versus the wait-list control arm.
h This study had 3 arms. For the purposes of our analysis, we compared the collective arm that combined both incentive groups to the control group.
i This study had 3 arms. For the purposes of our analysis, we compared the arm that received the intervention plus incentives to the control group.
j This study had 4 arms. For the purposes of our analysis, we compared the arm that received a combination of incentives to the control group.
k This study had 4 arms. For the purposes of our analysis, we compared the arm that received incentives for weight loss and self-monitoring to the control group.
| Author, year | Incentive description | Incentive typea | Monetary valueb | Recipientc | Frequencyd | Attainment certaintye | Schedulef |
|---|---|---|---|---|---|---|---|
| Almeida et al, 2015, (26) | Participants could receive cash based on percentage weight loss at quarterly weigh-ins (eg, 1% weight loss = $1.00, 2% weight loss = $2.00). Maximum potential earnings, ~$5 USD | Cash | Low | Individual | Multiple | Criteria-based guaranteed | Variable |
| Bennett et al, 2012 (27) | Participants could receive a grocery card ($50 USD) at completion of baseline, 6-, 12-, and 18-month visits and a grocery card ($75 USD) at 24 months. Participants also received a scale at 12-month visit and a blood pressure monitor at 18-month visit. Maximum potential earnings, $125 USD | Mixed | Low | Individual | Multiple | Guaranteed | Variable |
| Desai et al, 2020 (30) | Participants could receive incentives via a reloadable debit card for attendance and weight loss goals over the 12 months. Maximum potential earnings, $520 USD | Noncash financial | High | Mixed | Multiple | Criteria-Based Guaranteed | Variable |
| Dombrowski et al, 2020 (31) | At baseline, researchers deposited £400 GBP (~$550 USD) in a hypothetical bank account, and participants could secure/lose certain amounts when specific targets were reached/not reached. Maximum potential earnings, £400 GBP (~$550 USD) | Cash | High | Individual | Once | Criteria-Based Guaranteed | Variable |
| Faghri et al, 2014 (32) | Participants could receive cash for every 1 to 1.5 lb lost. Participants could choose 1 of 2 incentive plan: simple financial reward of $10 per lb of weight loss (maximum earnings, $260 USD) or simple financial reward plus deposit where participants could deposit $1–$5 per lb. of weight loss including a 1:1 match from the researchers. Maximum potential earnings, $340 USD | Cash | High | Individual | Once | Criteria-based guaranteed | Mixed |
| Finkelstein et al, 2017 (33) | Participants first paid a refundable fee to participate in the incentive plan. Participants could receive guaranteed cash payments or lottery cash prizes. Maximum potential earnings, S$600 SGD ($488 USD) | Cash | High | Individual | Multiple | Criteria-based guaranteed | Variable |
| John et al, 2011 (34) | Participants deposited their own money ($1–$3 per day) into a hypothetical account with a 1:1 match from the researchers. Incentive group A had a weight maintenance period weeks 25–32 and incentive group B did not. Maximum potential net earnings, $672 USD | Cash | High | Mixed | Multiple | Criteria-based guaranteed | Variable |
| Leahey et al, 2016 (29) | Participants could receive cash payments ($1–$10) weekly for submitting self-monitoring records and diet or activity information. An additional $25 was provided for maintaining weight loss. Maximum potential earnings, $185 USD | Cash | Low | Individual | Multiple | Criteria-based guaranteed | Variable |
| Leahey et al, 2015 (35) | Participants could receive cash payments ($1–$10) for submitting weight, nutrition, and activity information to be distributed after their 3-month assessment. Maximum potential earnings, $45 USD | Cash | Low | Individual | Once | Mixed | Variable |
| Morgan et al, 2011 (25) | Participants could receive sporting store gift vouchers (AU$50 [$37 USD]) per crew member based on the group that achieved the greatest mean weight loss and program completion. Maximum value, AU$100 (~$73 USD) | Noncash financial | Low | Group | Multiple | Criteria-based guaranteed | Fixed |
| Petry et al, 2011 (36) | Participants drew from a bowl with a chance to receive small incentives (healthy snacks, bottled water, toiletries) or large incentives ($20 gift cards, weight sets) worth $1–$100 USD for weight loss and completing weight loss activities. | Mixed | NA | Individual | Multiple | Criteria based lottery | Variable |
| Rounds et al, 2020 (37) | Participants could receive escalating incentives weekly for weight loss with a reset contingency if weekly weight loss goals were not met, starting at $4 USD in the first week. Maximum potential Earnings, $312 USD | Cash | High | Individual | Multiple | Criteria-based guaranteed | Variable |
| Shin et al, 2017 (38) | Participants could receive incentives for meeting daily physical activity goals and weight loss goals. Maximum potential earnings, ? 320,000 KRW (~$270 USD) | Cash | High | Individual | Multiple | Criteria-based guaranteed | Variable |
| Teychenne et al, 2015 (28) | Participants could receive motivational incentives such as a sports bag or water bottle. | Nonfinancial | NA | Individual | Multiple | Guaranteed | Fixed |
| VanEpps et al, 2019 (39) | Participants could receive incentives for attending group sessions and meeting weight loss goals. Maximum potential earnings, $240 USD | Cash | Low | Individual | Multiple | Criteria-based guaranteed | Fixed |
| Voils et al, 2020 (40) | Participants could receive incentives weekly (up to $30 USD per week on a reloadable debit card) for dietary self-monitoring and weight loss. Maximum potential earnings, $300 USD | Noncash financial | High | Individual | Multiple | Criteria-based guaranteed | Mixed |
| Volpp et al, 2008 (41) | Participants could receive incentives through a deposit contract or lottery incentive plan for meeting weight loss goals. The deposit contract required participants to deposit their own money ($1–$3) daily, which was matched 1:1 with an extra fixed payment of $3 per day and was refundable upon meeting or exceeding weight loss goals. The lottery plan offered a chance to receive daily incentives with a value of $3. Maximum net potential earnings, $168 USD | Cash | Low | Mixed | Multiple | Criteria-based guaranteed | Variable |
| West et al, 2020 (42) | Participants could receive incentives ($10–$15 per week via Amazon gift card) for submitting diet records and meeting weight loss goals. Maximum potential earnings, $230 USD | Noncash financial | Low | Individual | Multiple | Criteria-based guaranteed | Variable |
| Yancy et al, 2018 (43) | Participants could receive incentives through direct payments or lottery incentive plans for meeting weight loss goals. Maximum potential earnings, ~$590 USD | Cash | High | Individual | Multiple | Criteria-based guaranteed | Mixed |
Abbreviations: NA, not applicable; USD, US dollar; GBP, British pound sterling; SGD, Singapore dollar; AU, Australian dollar; KRW, South Korean won.
a The format of the incentive. Cash incentives were provided to participants in $USD currency or the currency of the study country’s location. Noncash financial incentives had a monetary value in a form other than currency (eg, gift cards, childcare or dependent assistance, transportation or store vouchers, health care premium discounts). Nonfinancial incentives were in kind and did not have a specific monetary value (eg, fitness equipment, products, various prizes). Mixed incentives were a combination of 2 or more incentive types.
b The worth of incentives provided to recipients for their participation in the program. A high amount, as defined by the authors, had a value of $270 or more. A low amount was defined as a value less than $270. This value was chosen because it is the median amount of money that participants could earn in 17 of the 19 studies included in our review.
c Who received the incentive: individuals, a group of individuals, or mixed (a combination of both).
d How often the incentive was provided to the recipient. Incentives could be given either once or multiple times throughout the intervention timeframe.
e How certain it was that a recipient would receive an incentive. Guaranteed = provided regardless of criteria being met; criteria-based guaranteed = must complete an activity or meet a milestone before the incentive is provided; criteria-based lottery = must complete an activity, task, or milestone to become eligible for an incentive lottery; lottery = an uncertain chance of receiving an incentive that could be based on completing an activity or meeting a milestone; mixed = a combination of 2 or more of these strategies.
f How the amount of the incentive was provided to recipients. Rates were based on what the recipient could potentially receive and were usually contingent on an activity, task, or timing. Fixed = the same incentive amount was given to recipients each time no matter what they did or achieved; variable = a varying incentive amount was provided to recipients over the intervention period; mixed = a combination of both.
| Incentive domain and subgroup | N (mean difference) [95% CI] | P Valuea | I2 (%) | Difference between subgroupsb |
|---|---|---|---|---|
| Weight | ||||
| Typec | ||||
| Cash | 12 (−1.79) [−2.53 to −1.05] | <.001 | 42.14 | Q = 0.15, P = .69 |
| Other types | 7 (−2.03) [−3.01 to −1.06] | <.001 | 64.85 | |
| Monetary valued | ||||
| High | 9 (−2.04) [−2.77 to −1.32] | <.001 | 0.00 | Q = 0.00, P = .95 |
| Low | 8 (−2.01) [−3.05 to −0.96] | <.001 | 73.93 | |
| Attainment certaintye | ||||
| Criteria-based guaranteed | 15 (−2.20) [−3.01 to −1.40] | <.001 | 57.59 | Q = 4.92, P = .03 |
| Other | 4 (−1.15) [−1.63 to −0.66] | <.001 | 0.00 | |
| Schedulef | ||||
| Fixed | 3 (−2.02) [−4.00 to −0.03] | .046 | 84.15 | Q = 0.04, P = .83 |
| Other schedules | 16 (−1.80) [−2.39 to −1.20] | <.001 | 34.97 | |
| Body mass index (weight in kg divided by height in m2) | ||||
| Typec | ||||
| Cash | 4 (−0.44) [−0.86 to −0.03] | .034 | 47.98 | Q = 0.14, P = .71 |
| Other types | 3 (−0.55) [−0.93 to −0.17] | .005 | 64.51 | |
| Monetary Valued | ||||
| High | 3 (−0.65) [−1.12 to −0.19] | .010 | 6.81 | Q = 0.20, P = .65 |
| Low | 3 (−0.50) [−0.99 to −0.00] | .048 | 76.73 | |
| Attainment certaintye | ||||
| Criteria-based guaranteed | 5 −0.69 [−1.21 to −0.18] | .008 | 68.67 | Q = 1.15, P = .28 |
| Other | 2 (−0.39) [−0.57 to −0.22] | <.001 | 0.00 | |
| Schedulef | ||||
| Fixed | 2 (−0.82) [−1.79 to −0.15] | .097 | 81.84 | Q = 0.77, P = .38 |
| Other schedules | 5 (−0.37) [−0.64 to −0.10] | .007 | 33.58 | |
Abbreviations: Q, Cochrane Q statistic.
a Refers to whether the association between the incentive domain subgroup and the health indicator was significant, based on the z value in the mixed-effects analysis.
b Refers to whether the mean differences for the 2 subgroups were statistically different from each other, based on the Q value in the mixed-effects analysis.
c Cash is currency provided to participants; other types include noncash financial, nonfinancial, or mixed.
d High includes studies that provided incentives valued at $270 or more; low includes studies that provided incentives valued at less than $270. For weight, the Petry et al (36) and Teychenne et al (28) studies were not included in the moderator analysis because they did not have a cash value. For BMI, the Teychenne et al (28) study was not included because it did not have a cash value.
e Criteria-based guaranteed includes studies where an activity, task, or milestone must have been met before incentives were provided; other includes guaranteed (incentive provided regardless of criteria being met), criteria-based lottery (an activity, task, or milestone must be completed to be eligible for an incentive lottery), and mixed (a combination of 2 or more of these strategies).
f Fixed means the same incentive amount was given to recipients each time, no matter what they did or achieved; other schedules include variable (a varying incentive amount was given to recipients over the intervention period) and mixed (a combination of both schedules).
Appendix
| Database | Strategy |
|---|---|
| Medline (OVID) 1946– | (money OR cash OR incentiv* OR token econom* OR token reinforcement* OR payment* OR paid OR earn* OR reimburse* OR wage* OR contingency management OR coupon* OR discount OR voucher* OR gift* OR free food OR prize* OR award* OR reward*).mp. AND(Weight reduction OR weight management OR weight loss* OR obesity OR overweight OR body weight OR body mass OR bmi OR excess weight OR diet* OR feeding behavior OR eating OR nutrition OR lifestyle OR life style OR behavio?r change OR behavior modification OR behavior therapy OR health promotion OR health behavior OR healthy OR fitness OR exercise OR exercising OR physical activit* OR strength training OR diabetes OR diabetic OR prediabet* OR pre-diabet* OR glucose OR glycemic OR glycemia OR hyperglycemia OR glycated hemoglobin OR hypertensi* OR blood pressure OR cholesterol OR HbA1c).mp. AND (trial* OR randomized OR randomly OR rct* OR control group* OR clinical stud*).ti,ab. OR randomized controlled trial.ptLimit 2008 – ; English |
| Embase (OVID) 1988– | (money OR cash OR incentiv* OR token econom* OR token reinforcement* OR payment* OR paid OR earn* OR reimburse* OR wage* OR contingency management OR coupon* OR discount* OR voucher* OR gift* OR free food OR prize* OR award* OR reward*).mp. AND(Weight reduction OR weight management OR weight loss* OR obesity OR overweight OR body weight OR body mass OR bmi OR excess weight OR diet* OR feeding behavio?r OR eating OR nutrition OR lifestyle OR life style OR behavio?r change OR behavio?r modification OR behavio?r therapy OR health promotion OR health behavio?r OR healthy OR fitness OR exercise OR exercising OR physical activit* OR strength training OR diabetes OR diabetic OR prediabet* OR pre-diabet* OR glucose OR glycemic OR glycemia OR hyperglycemia OR glycated hemoglobin OR hypertensi* OR blood pressure OR cholesterol OR HbA1c).mp. AND (trial* OR randomi?ed OR randomly OR rct* OR control group* OR clinical stud*)Limit 2008 – ; English ; exclude Medline journals |
| PsycINFO (OVID) 1806– | (money OR cash OR incentiv* OR token econom* OR token reinforcement* OR payment* OR paid OR earn* OR reimburse* OR wage* OR contingency management OR coupon* OR discount* OR voucher* OR gift* OR free food OR prize* OR award* OR reward*).mp. AND(Weight reduction OR weight management OR weight loss* OR obesity OR overweight OR body weight OR body mass OR bmi OR excess weight OR diet* OR feeding behavio?r OR eating OR nutrition OR lifestyle OR life style OR behavio?r change OR behavio?r modification OR behavio?r therapy OR health promotion OR health behavio?r OR healthy OR fitness OR exercise OR exercising OR physical activit* OR strength training OR diabetes OR diabetic OR prediabet* OR pre-diabet* OR glucose OR glycemic OR glycemia OR hyperglycemia OR glycated hemoglobin OR hypertensi* OR blood pressure OR cholesterol OR HbA1c).mp. AND (trial* OR randomi?ed OR randomly OR rct* OR control group* OR clinical stud*) Limit 2008 – ; English |
| Cochrane Library | (money OR cash OR incentiv* OR “token econom*” OR “token reinforcement*” OR payment* OR paid OR earn* OR reimburse* OR wage* OR “contingency management” OR coupon* OR discount OR voucher* OR gift* OR “free food” OR prize* OR award* OR reward*):ti AND(“Weight reduction” OR “weight management” OR “weight loss*” OR obesity OR overweight OR “body weight” OR “body mass” OR bmi OR “excess weight” OR diet* OR “feeding behavio?r” OR eating OR nutrition OR lifestyle OR “life style” OR “behavio?r change” OR “behavio?r modification” OR “behavio?r therapy” OR “health promotion” OR “health behavio?r” OR healthy OR fitness OR exercise OR exercising OR “physical activit*” OR “strength training” OR diabetes OR diabetic OR prediabet* OR pre-diabet* OR glucose OR glycemic OR glycemia OR hyperglycemia OR “glycated hemoglobin” OR hypertensi* OR “blood pressure” OR cholesterol OR HbA1c):ti,abLimit 2008 – ; English |
a Duplicates were identified using the EndNote (Clarivate) automated “find duplicates” function with preference set to match on title, author and year, and removed from your EndNote library. Additional duplicates will likely be found that EndNote was unable to detect.
Post-Test Information
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/pcd. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers.
You must be a registered user on http://www.medscape.org. If you are not registered on http://www.medscape.org, please click on the “Register” link on the right hand side of the website.
Only one answer is correct for each question. Once you successfully answer all post-test questions, you will be able to view and/or print your certificate. For questions regarding this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@medscape.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please go to https://www.ama-assn.org. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate, and present it to your national medical association for review.
Post-Test Questions
Study Title: Effectiveness of Incentives for Improving Diabetes-Related Health Indicators in Chronic Disease Lifestyle Modification Programs: a Systematic Review and Meta-Analysis
CME Questions
- What was the most common patient incentive employed in the 19 randomized trials included in the current meta-analysis?
- Gift cards
- Coupons
- Cash
- Free transportation
- Which one of the following statements regarding the effect of patient incentives on body weight/body mass index (BMI) in the current study is most accurate?
- Compared with the control group, the incentive group lost an average of 6 kg and experienced a decline in BMI of 2 mg/kg2
- Compared with the control group, the incentive group lost an average of 2 kg and experienced a decline in BMI of 0.5 mg/kg2
- Incentives had no effect on body weight or BMI
- Participants in the incentive groups experienced a slight increase in body weight and BMI compared with the control group
- How did patient incentives affect blood pressure values in the current study?
- Incentives failed to improve either systolic or diastolic blood pressure
- Incentives improved systolic, but not diastolic, blood pressure
- Incentives improved diastolic, but not systolic, blood pressure
- Incentives improved both systolic and diastolic blood pressure
- Which one of the other following cardiometabolic variables was most improved with patient incentives in the current study?
- Total cholesterol
- Waist circumference
- Average daily steps on a pedometer
- HbA1c
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions.