Tuberculosis Technical Instructions for Civil Surgeons
- Background
- Tuberculosis Screening Summary
- Medical History
- Physical Exam
- Immune Response to M. tuberculosis Antigens
- Chest Radiography
- Infectious Tuberculosis Disease and Required Referral to Health Departments
- Latent Tuberculosis Infection and Required Reporting to Health Departments
- Tuberculosis Laboratory Testing by the Health Department
- Extrapulmonary Tuberculosis
- Tuberculosis Treatment
- The eMedical System Documentation
- Tuberculosis Classifications
- People with a past history of a Tuberculosis Condition
- Re-classification of Applicants after Treatment for Infectious Tuberculosis Disease
- Waivers
- Glossary of Abbreviations
- Definitions of Selected Terms
At a Glance
These instructions are in accordance with CDC regulations and are for the use of civil surgeons evaluating persons applying for adjustment of status for U.S. permanent residence and others required to have a medical examination.
Background
The CDC Division of Global Migration Health (DGMH) developed these instructions in consultation with U.S. tuberculosis subject matter experts. One of the goals of the status adjustment medical examination is to diagnose and treat certain infectious diseases, thus these instructions define the specific responsibilities of civil surgeons in terms of testing for infectious tuberculosis disease among applicants and referral for treatment. For the purposes of these instructions, the term infectious tuberculosis disease refers to disease of the lung parenchyma, pleura, larynx, or intrathoracic lymph nodes. Other forms of extrapulmonary tuberculosis and latent tuberculosis infection (LTBI) are not included in the definition of infectious tuberculosis disease and are defined separately.
These instructions are specific to the status adjustment medical examination and should not be used as guidance to test for or treat tuberculosis disease in other settings or as a clinical manual that defines detailed laboratory procedures or specific treatment regimens. Treatment of applicants for drug-susceptible tuberculosis disease must be consistent with current CDC guidance: Treatment for TB Disease.
U.S. Citizenship and Immigration Services (USCIS) in the U.S. Department of Homeland Security (DHS) designates civil surgeons. Civil surgeons must perform the medical examination according to the procedures prescribed in these Technical Instructions. Civil surgeons must report all confirmed or suspected tuberculosis disease cases promptly to the health department of jurisdiction to ensure that applicants are evaluated for infectious tuberculosis disease, started on the appropriate drug regimens, if indicated, and that thorough contact or source case (for pediatric applicants) investigations are initiated when needed.
The instructions in this document supersede all previous Tuberculosis Technical Instructions, Updates to the Technical Instructions, and communications to civil surgeons. These instructions are to be followed for infectious tuberculosis disease screening and treatment of all applicants. These instructions go into effect March 11, 2024.
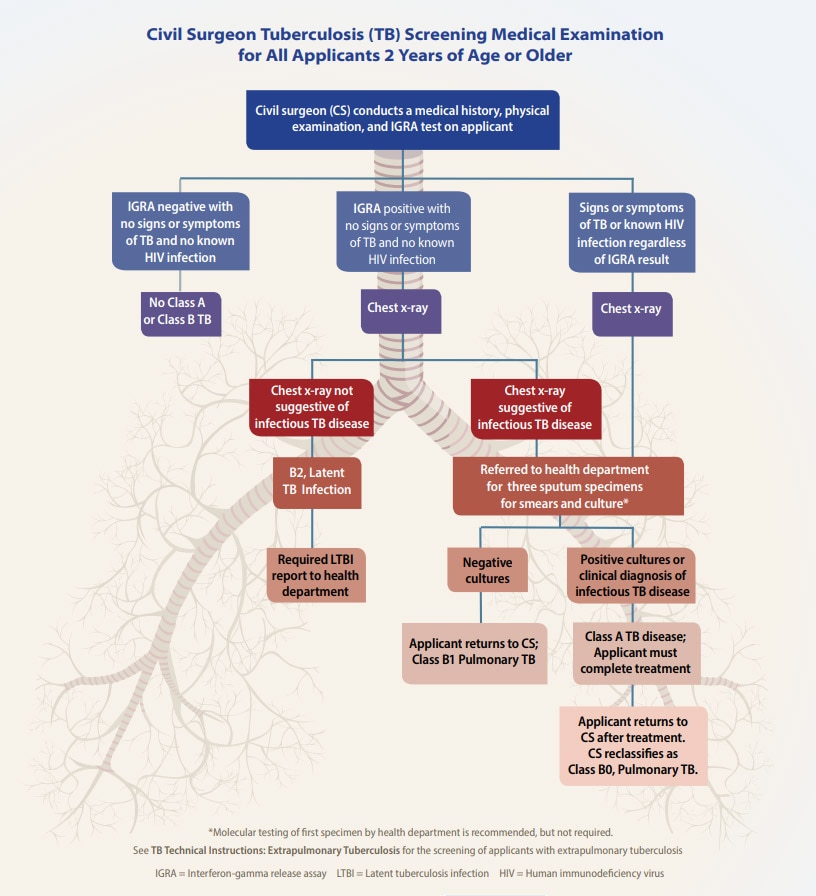
Figure 1. Civil Surgeon Tuberculosis (TB) Screening Medical Examination for All Applicants 2 Years of Age or Older
All applicants ≥2 years of age must have an interferon-gamma release assay (IGRA).
All applicants with a positive IGRA, known human immunodeficiency virus (HIV) infection, or signs or symptoms of tuberculosis disease, must have a chest x-ray.
Human immunodeficiency virus (HIV) testing is not a requirement of the U.S. medical examination; however, civil surgeons may advise applicants for whom testing is clinically indicated about HIV testing.
A complete screening medical examination for infectious tuberculosis disease consists of a medical history, physical examination, interferon-gamma release assay (IGRA) if 2 years old or older, chest x-ray when required, and referral to the health department of jurisdiction to evaluate for infectious tuberculosis disease when required.
All applicants ≥2 years of age must have an IGRA performed. Current U.S. clinical practice guidelines [PDF – 5 pages] suggest using tuberculin skin test (TST) rather than an IGRA in healthy children <5 years of age; some pediatric experts use IGRA for younger children. Because of programmatic concerns in the setting of this examination, civil surgeons must use an IGRA as defined in these instructions for all applicants ≥2 years of age.
If the IGRA is positive or if the applicant has signs or symptoms of tuberculosis disease or has known human immunodeficiency virus (HIV) infection, a chest x-ray (anteroposterior or posteroanterior view and a lateral view for applicants <10 years of age; posteroanterior view for applicants ≥10 years of age) must be performed. Applicants who have chest x-ray findings suggestive of infectious tuberculosis disease, signs or symptoms of infectious tuberculosis disease, or known HIV infection must be referred to the health department of jurisdiction for sputum testing.
All applicants <2 years of age must have a physical examination and history provided by a parent or responsible adult who knows the child best. Those applicants who have signs or symptoms suggestive of tuberculosis disease or have known HIV infection must have a TST or IGRA, must have a chest x-ray (anteroposterior or posteroanterior view and a lateral view) regardless of TST or IGRA results, and must be referred to the health department of jurisdiction for further evaluation.
HIV testing is not a requirement of the medical screening process; however, civil surgeons may advise applicants for whom testing is clinically indicated about HIV testing. Such applicants may include those with signs and symptoms suggestive of HIV infection or those with tuberculosis disease. For such applicants, the consent for HIV testing must include the following:
- Applicants understand they do not have to be tested for HIV.
- Applicants understand that if they would like to be tested for HIV, they do not have to be tested for HIV by a civil surgeon.
- Applicants understand that civil surgeons must include the test results on the paperwork they complete.
People with HIV infection are less likely to have an abnormal chest x-ray or positive IGRA during infectious tuberculosis disease, and negative IGRA results do not rule out infectious tuberculosis disease; thus, all applicants with known HIV infection must provide sputum specimens for smears and culture, regardless of IGRA or chest x-ray results, to rule out infectious tuberculosis disease.
Civil surgeons must not refer applicants to a health department for IGRA testing or chest x-ray; all IGRAs and chest x-rays ordered by civil surgeons must be performed independently of a health department. The only exception is for civil surgeons who work at a health department.
If an applicant has a positive IGRA, but has no signs or symptoms of tuberculosis disease, a negative chest x-ray, and no known HIV infection, the applicant must receive a classification of Class B2 TB, Latent TB Infection and must be reported to the health department of jurisdiction. If the applicant has a documented history of completing treatment for tuberculosis disease or LTBI, the chest x-ray is negative, and they have no signs or symptoms of tuberculosis and no known HIV infection, they can receive a classification of “No TB Classification,”
Each aspect of the examination for tuberculosis is detailed below.
- The medical history should focus on risk factors for infectious tuberculosis disease, including previous history of tuberculosis disease; illness suggestive of tuberculosis disease (such as cough of >3 weeks’ duration, dyspnea, weight loss, fever, or hemoptysis); prior treatment suggestive of tuberculosis disease treatment; and prior diagnostic evaluation suggestive of tuberculosis disease. The clinical expression of infectious tuberculosis disease may be different in children than in adults, and for children may only include generalized findings such as fever, night sweats, growth delay, and weight loss. Children are also more prone to extrapulmonary tuberculosis, such as meningitis, and disease of the middle ear and mastoid, lymph nodes, bones, joints, and skin.
- If the applicant was previously diagnosed with tuberculosis disease in the United States, the civil surgeon should try to get those records by contacting the state or local health department of jurisdiction for a copy of the applicant’s medical records or asking the applicant to obtain and provide those records.
- The medical history must also include inquiries regarding family or household contact with a person who has or had tuberculosis disease, or an illness, treatment, or diagnostic evaluation suggestive of tuberculosis disease.
Pertinent elements of the physical examination for infectious tuberculosis disease include general characteristics such as height, weight, temperature, heart rate, respiratory rate, and blood pressure; a thorough pulmonary examination; inspection and palpation of lymph nodes; and inspection for scars of scrofula or prior chest surgery.
All applicants 2 years of age or older must have an IGRA test to determine immune response to M. tuberculosis antigens.
Exceptions include applicants with written documentation from a physician of a previous positive IGRA. For past positive IGRA results, the written documentation must include date of the test, type of IGRA performed, test results in standard units of measurement, the test interpretation (i.e., positive), and the testing physician’s name, signature, and office information. Applicants ≥2 years of age who provide documentation of a previous positive TST must still have an IGRA performed; if the IGRA is negative, the applicant is considered to have a negative immune response to M. tuberculosis antigens in this examination.
- IGRA – CDC will only allow use of IGRA tests approved by the U.S. Food and Drug Administration (FDA). Currently, there are two FDA-approved products: QIAGEN QuantiFERON® (any iteration approved by FDA) or Oxford Immunotec T-SPOT®.TB. Civil surgeons must follow the manufacturers’ written instructions for collection of samples, performing testing, and interpreting test results. For the purpose of tuberculosis screening according to these Technical Instructions, an indeterminate test result must be documented as indeterminate and not result in repeat testing by the civil surgeon, chest x-ray, or B2 classification. Although not required, applicants with an indeterminate test result should be advised to have a repeat test for their own health benefit. The IGRA test used and the results must be documented in Part 8, A. 1 of the I-693 [PDF- 14 pages], even for those with negative or indeterminate results. Results should be available within 72 hours of sample collection.
- TST – TST can only be used in children <2 years of age when indicated. Tuberculin purified protein derivative (PPD) must be administered intradermally by the Mantoux method. Ideally, preparations used should be equivalent to 5TU PPD-S. A TST is considered positive if it results in induration ≥10 mm (≥5 mm if applicant is HIV-positive or a known contact to a person with tuberculosis disease).
Civil surgeons must refer applicants with abnormal chest x-rays suggestive of infectious tuberculosis disease to the health department of jurisdiction for further evaluation.
Although findings on a single chest x-ray cannot confirm the diagnosis of infectious tuberculosis disease, they do determine the need for further evaluation by the health department of jurisdiction.
A chest radiograph (chest x-ray) is required for all applicants who:
- Have a positive IGRA result; or
- Have known HIV infection, regardless of IGRA result; or
- Have signs or symptoms of tuberculosis disease, regardless of IGRA result; or
- Have extrapulmonary tuberculosis, regardless of IGRA result
When performed, chest x-rays must consist of a standard posteroanterior view for all applicants ≥10 years of age. Applicants <10 years of age who receive a chest x-ray must have a standard anteroposterior or standard posteroanterior view and must also have a lateral view. The chest x-ray must be labeled “PA” or “AP” for the benefit of the radiologist’s review. Additional views can be taken if clinically appropriate.
The civil surgeon must indicate on the chest x-ray requisition that there is a high suspicion of infectious tuberculosis disease. Chest x-rays must be interpreted by a radiologist and reviewed by the civil surgeon. Documentation of the results should be available same day but must be available no later than 3 days from the time they were performed. Chest x-rays of any applicants must be retaken if the initial chest x-ray is suboptimal because of factors such as poor inspiration or motion artifact. Chest x-ray interpretations must include comparisons with prior chest x-rays, if available.
Chest x-ray findings that are suggestive of infectious tuberculosis disease and require referral to the health department for sputum specimen collection include:
- Infiltrate or consolidation
- Reticular markings suggestive of fibrosis
- Cavitary lesion(s)
- Nodule(s) or mass(es) with poorly defined margins (such as tuberculoma)
- Pleural effusion
- Hilar/mediastinal adenopathy
- Miliary findings
- Discrete linear opacity
- Discrete nodule(s) without calcification
- Volume loss or retraction
- Irregular thick pleural reaction
- Other findings suggestive of tuberculosis disease per radiologist’s interpretation
Chest x-ray findings that do not require referral to the health department for sputum specimen collection include:
- Cardiac abnormalities
- Musculoskeletal abnormalities
- Smooth pleural thickening, confirmed not to be an effusion
- Diaphragmatic tenting
- Single or scattered calcified pulmonary nodule(s)
- Calcified lymph node(s)
If a chest x-ray is required, applicants with clinical and radiographic findings suggestive of common bacterial infections of the respiratory tract may be treated with a course of antibiotics. However, fluoroquinolones must not be used for empiric treatment of respiratory infections because they are a mainstay of second-line therapy for tuberculosis disease and their use could both result in mistreatment of tuberculosis disease and lead to drug-resistant tuberculosis disease. After treatment for respiratory tract infections, the chest x-ray for medical screening should not be performed until at least 8 weeks after treatment unless the applicant’s clinical status warrants further evaluation earlier than 8 weeks after treatment.
Applicants who are pregnant and have a positive IGRA or any of the other criteria listed above are required to have a chest x-ray to adjust status. Applicants who are pregnant may postpone the required chest x-ray (and status adjustment medical examination) until after pregnancy but are required to have a chest x-ray to adjust status if they meet one of the above criteria. If Civil Surgeons choose to obtain consent from pregnant women for the chest x-ray, they should develop their own consent form. Lead shielding is no longer recommended or required for the status adjustment medical examination for any applicants, including those who are pregnant.
The radiologist must use digital radiography (computed radiography [CR] or direct digital radiography [DDR]) to obtain plain chest x-rays of applicants. Digitized analog images are not acceptable.
Digital radiography equipment systems must meet the following requirements:
- Images must be interpreted by a radiologist on a high-resolution screen. The screens used by the radiologists must be medical-grade monitors that are at least 3 megapixels (MP) in “display resolution” AND that are advertised as being appropriate for primary image interpretation (not for image review). The screens used by the civil surgeons to review the images are not required to meet this standard.
- Images must not be interpreted from laser-printed films, as the quality of printing varies greatly and film format cannot be optimized.
- DICOM images at least 4-5 megabytes (MB) in size must be provided to health departments to which referrals are made for evaluation for infectious tuberculosis disease or LTBI is reported.
The health department of jurisdiction will determine whether the applicant has infectious tuberculosis disease and needs treatment.
Applicants with suspected infectious tuberculosis disease must receive their treatment from providers with considerable experience and expertise with tuberculosis, such as health departments or expert clinicians under contract to, or designated by, the health departments. U.S. public health departments have considerable experience in dealing with such difficult issues such as patient non-adherence, drug resistance, and HIV co-infection; and most use directly observed therapy (DOT) to ensure that people with infectious tuberculosis disease continue their therapy until completion.
For applicants requiring referral for suspected infectious tuberculosis disease, the civil surgeon must not classify, issue medical clearance for tuberculosis, sign the I-693 [PDF – 14 pages] form, or submit the eMedical record until the applicants return from the local health department with documentation of the results of their infectious tuberculosis disease evaluation.
Specimens must be incubated for a minimum of 6 weeks for liquid cultures and 8 weeks for solid cultures, before a final negative report can be issued and the applicant can receive a Class B1, Pulmonary TB classification.
Positive cultures or clinically diagnosed tuberculosis disease will result in a Class A TB Classification.
All applicants with an abnormal chest x-ray suggestive of infectious tuberculosis disease must be referred to the health department of jurisdiction for further evaluation. Applicants with clinical signs or symptoms suggestive of infectious tuberculosis disease or known HIV infection must also be referred regardless of IGRA result or chest x-ray findings.
All applicants with extrapulmonary tuberculosis disease must also be referred to the health department for further evaluation regardless of chest x-ray results.
At the time of referral, include the IGRA result, chest x-ray report and images (as specified above), description of any signs or symptoms, the approximate date of U.S. arrival, and the reason for referral.
If the applicant appears very ill and tuberculosis disease is suspected, the referral must be made immediately to avoid delay in treatment, and urgent hospitalization can occur before referral if clinically indicated.
Latent Tuberculosis Infection and Required Reporting to Health Departments
All applicants diagnosed with latent tuberculosis infection (LTBI) must be reported to the local health department.
Applicants with a positive IGRA result and chest x-ray not suggestive of infectious tuberculosis disease, no known HIV infection, no signs or symptoms of infectious tuberculosis disease, no evidence of extrapulmonary tuberculosis disease, and no documentation of completing treatment for tuberculosis disease or LTBI, have LTBI. LTBI will typically present in a person without HIV as a positive IGRA with a negative chest x-ray and no signs or symptoms of infectious or extrapulmonary tuberculosis disease. LTBI is not an infectious condition and therefore treatment is not required for the purposes of status adjustment. The positive IGRA results and LTBI diagnosis must be communicated to the applicant. The applicant’s name, contact information, IGRA results, and chest x-ray results must be reported to the local health department of jurisdiction. Nationwide, health departments have different systems for managing LTBI. For this reason, civil surgeons should communicate proactively with the health department of jurisdiction to coordinate reporting. If the health department agrees, the eMedical reporting can serve as this LTBI report.
LTBI diagnosis requires a report to the health department, not a referral, and these applicants are not required to be evaluated by the health department. The I-693 can be completed and given to the applicant before this report is made. Applicants with LTBI must also be entered into eMedical. Civil surgeons must inform such applicants that their LTBI diagnosis will be reported to the health department and must advise the applicant that treatment is important to prevent infectious tuberculosis disease and protect the applicant’s health as well as the health of their families and communities, although not required to complete the status adjustment process. After the I-693 is signed, the civil surgeon can offer these applicants treatment for LTBI or refer them for treatment elsewhere.
Applicants who have documentation of being diagnosed with and completing treatment for LTBI prior to the civil surgeon examination must have a chest x-ray as part of the civil surgeon evaluation. If the chest x-ray is negative and the applicant does not have signs or symptoms of infectious tuberculosis disease or known HIV infection, the applicant does not have to be diagnosed with LTBI or reported to the health department and can be classified as “No Class A or Class B TB.”
All sputum laboratory work must be performed by the local or state health department or by a private laboratory designated by the health department. An applicant with an abnormal chest x-ray suggestive of infectious tuberculosis disease, signs or symptoms of infectious tuberculosis disease, or known HIV infection must provide three early morning fasting sputum specimens consisting of 5–10 mL each; specimens must be collected at least 24 hours apart, preferably on consecutive working days. The collection of the three sputa must be supervised by a public health official, health-care worker, or laboratory technician.
All three sputum specimens must be examined for the presence of acid-fast bacilli (AFB) and cultured for mycobacteria and confirmation of the Mycobacterium species, at least to the M. tuberculosis complex level. Positive M. tuberculosis cultures must undergo drug susceptibility testing (DST). When sputum testing is required, the health department must not sign the I-693 referral section until cultures are reported as negative at the end of the incubation period, or after completion of tuberculosis disease treatment with two negative end-of-treatment culture results for those diagnosed with tuberculosis disease.
Molecular testing
Performing a molecular test, defined as a diagnostic nucleic acid amplification test (NAAT), is recommended on the first sputum specimen from applicants who require sputum testing. If the molecular test is positive, the applicant can begin tuberculosis treatment based on these results. However, a negative molecular test cannot be used to rule out infectious tuberculosis disease, and the applicant must wait for culture results. If the molecular test is negative, and the cultures are positive, the applicant must be diagnosed with Class A, Infectious Tuberculosis Disease. If the molecular test is positive and the cultures are negative, the health department physician should use their clinical judgement in diagnosing the patient.
Extrapulmonary Tuberculosis
Applicants diagnosed with extrapulmonary tuberculosis only must have a chest x-ray ordered by the civil surgeon and must be referred to the health department for three sputum specimens for smears and culture, regardless of chest x-ray results.
If the chest x-ray is suggestive of infectious tuberculosis disease, these applicants are Class A TB and must complete tuberculosis disease treatment even if sputum smears and cultures are negative.
If the chest x-ray is normal, and the sputum smears and cultures are negative, the applicant can be assigned a Class B1 TB, Extrapulmonary tuberculosis classification and the I-693 completed.
Any applicant diagnosed with infectious tuberculosis disease must receive a classification of Class A TB and is not cleared until successful completion of treatment, regardless of the diagnostic criteria.
If the health department of jurisdiction diagnoses infectious tuberculosis disease (either laboratory confirmed or clinically), the health department must treat and manage the applicant’s infectious tuberculosis disease. Health departments are responsible for ensuring that people with infectious tuberculosis disease in their jurisdictions are started promptly on and complete appropriate drug regimens, and for conducting thorough contact investigations.
A civil surgeon who wishes to treat a referred applicant for diagnosed tuberculosis disease must do so in close collaboration and consultation with the health department of jurisdiction. If a civil surgeon serves as the treating physician, treatment must be delivered as DOT.
Applicants with drug-susceptible infectious tuberculosis disease must be treated according to CDC guidance: Treatment for TB Disease.
The eMedical System Documentation
The civil surgeon is responsible for assigning a tuberculosis classification for each applicant. After a final tuberculosis classification has been made and the I-693 has been completed, civil surgeons are required to enter applicants with the following tuberculosis classifications into eMedical: Class B0, B1 Pulmonary or Extrapulmonary, and B2 Latent TB Infection. Records must be entered no later than 5 business days after the civil surgeon completes and signs the I-693 form; eMedical entries do not replace any portion of the I-693. Civil surgeons must upload a copy of the PDF I-693 form and if available, a copy of the chest x-ray and the IGRA results. Only applicants with a completed and signed I-693 form should have their examination records submitted into eMedical. See webinars for eMedical instructions.
Applicants diagnosed with infectious tuberculosis disease by the health department will need to return to the civil surgeon after treatment so they can be reclassified and cleared and the I-693 and eMedical record can be completed. Class A will typically not be a final tuberculosis classification, because after treatment is completed, the classification will change to Class B0. Health departments will not find information in the Electronic Disease Notification (EDN) system about applicants referred to them for infectious tuberculosis disease because these cases are still in progress and entry into eMedical (the source of EDN data) occurs after classification completion. Therefore, civil surgeons and the health department will first need to document evaluation and treatment of Class A applicants using the paper I-693 form.
For applicants requiring referral for infectious tuberculosis disease evaluation, the civil surgeon must not classify, issue medical clearance for tuberculosis, or sign the I-693 form until the applicants return from the local health department with documentation of the results of their infectious tuberculosis disease evaluation.
Solid tuberculosis cultures require 8 weeks of incubation before results can be reported as negative and the applicant can receive a Class B1, Pulmonary TB classification or a Class B0 classification for those who have completed treatment. Positive cultures or clinically diagnosed infectious tuberculosis disease will result in a Class A TB Classification until the applicant has completed treatment and is reclassified as B0.
The following are the tuberculosis classification options:
Applicants without clinical findings of infectious tuberculosis disease or extrapulmonary disease, without known HIV infection, and with a negative IGRA. For people with a past history of a tuberculosis condition, see the section below for classification instructions.
These applicants do NOT have to be entered into eMedical.
Class A TB
All applicants who have infectious tuberculosis disease. All applicants with 1 or more cultures positive for M. tuberculosis complex must be diagnosed with infectious tuberculosis disease regardless of other test results or clinical findings. Infectious tuberculosis disease can also be diagnosed clinically based on signs and symptoms, or diagnosed based on positive molecular test results. This class includes applicants who are diagnosed with infectious tuberculosis disease by the civil surgeon and health department and applicants who present to the civil surgeon already on tuberculosis treatment at the time of their medical exam. This class also includes applicants with extrapulmonary tuberculosis who have a chest x-ray suggestive of tuberculosis disease, regardless of sputum smear and culture results.
Class B0, Pulmonary TB
Applicants who were diagnosed with infectious tuberculosis disease by the civil surgeon and health department during the medical examination process, or presented to the civil surgeon already on treatment and successfully completed treatment for infectious tuberculosis disease.
Class B1, Pulmonary TB
Applicants who have signs or symptoms, physical exam, or chest x-ray findings suggestive of infectious tuberculosis disease; or have known HIV infection; are referred to the health department for additional evaluation; but have negative sputum cultures and are not diagnosed with infectious tuberculosis disease.
Class B1, Extrapulmonary TB
Applicants with extrapulmonary tuberculosis with a normal chest x-ray and negative sputum cultures.
Class B2 TB, Latent TB Infection
Applicants who have a positive IGRA or documented history of a positive IGRA (unless applicant has documentation of having previously completed treatment for LTBI or tuberculosis disease, see section below), and a chest x-ray not suggestive of infectious tuberculosis disease. The IGRA result, the applicant’s status with respect to treatment, and the medication(s) used (if treated) must be documented. For applicants who had more than one IGRA, all dates and results must be documented. All of these applicants must be reported to the health department of jurisdiction. The civil surgeon can treat these applicants for LTBI or refer them for treatment elsewhere, but the applicants do not have to complete treatment before they are medically cleared and their I-693 forms are completed because LTBI is not a Class A condition.
Class B, Other Chest Condition (non-TB)
Applicants with an abnormal chest x-ray suggestive of disease that is not tuberculosis, provided the applicant has no clinical signs or symptoms of infectious or extrapulmonary tuberculosis disease or known HIV infection and has a negative IGRA.
People with a past history of a Tuberculosis Condition
It is possible to have infectious tuberculosis disease more than once, and it is possible to develop infectious tuberculosis after appropriate treatment for LTBI. Thus, people with these histories must be screened and have specific considerations:
- Applicants with a history of positive IGRA must have a chest x-ray. If the chest x-ray is normal, the applicant has no signs or symptoms of tuberculosis disease and no known HIV infection, the civil surgeon must report that the applicant has LTBI to the health department of jurisdiction, unless the applicant has documentation of having previously completed treatment for LTBI.
- Applicants who have documentation of previous diagnosis and complete treatment for LTBI and who have a negative chest x-ray, no signs or symptoms of tuberculosis disease, and no known HIV infection, do not have to be diagnosed with LTBI or reported to the health department and can be classified as “No Class A or Class B TB.”
- Applicants who have documentation of a previous diagnosis of and complete treatment for LTBI, and who have an abnormal chest x-ray or signs or symptoms of infectious tuberculosis disease at the time of the medical examination, or known HIV infection, must have sputum collection performed.
- Applicants with a well-documented history of completion of tuberculosis disease treatment who have a positive or negative IGRA, a negative chest x-ray, no current signs or symptoms of tuberculosis disease, and no known HIV infection are also assigned “No Class A or Class B TB.”
- Applicants who have a self-reported history of completion of tuberculosis disease treatment without documentation should be evaluated as if they have not had tuberculosis disease. They must begin with an IGRA, and if it is negative, and they have no signs or symptoms of infectious tuberculosis disease and no known HIV infection, then no further workup is needed.
- People with a history of treated infectious tuberculosis disease may have residual DNA from dead tuberculosis organisms, that can cause a false positive result on molecular tests. The health department physician should take this into consideration and use clinical judgment when interpreting results for these applicants to determine if this represents new or past infection.
Tuberculosis screening tests, IGRA, and radiography must be ordered and completed at the time of the medical examination. The examination is essentially a “snapshot in time” and the tests must reflect the applicant within that snapshot and must also be performed with safeguards to prevent fraud. All components of the medical examination completed by a civil surgeon must be less than 1 year old at the time the civil surgeon signs the I-693. If the results are more than a year old, the examination components will need to be repeated by the civil surgeon.
Exceptions include applicants with written documentation from a physician of a previous positive IGRA. For past positive IGRA results, the written documentation must include date of the test, type of IGRA performed, test results in standard units of measurement, the test interpretation (i.e., positive), and the testing physician’s name, signature, and office information.
An applicant with infectious tuberculosis disease must complete a recommended course of tuberculosis disease treatment per CDC guidelines found here for drug-susceptible tuberculosis disease: Treatment for TB Disease and here for drug-resistant tuberculosis disease: Drug-Resistant TB. When treatment for infectious tuberculosis disease has been completed, a representative of the health department must sign the “Referral Evaluation” section (Part 9) of the I-693 form, indicating that the applicant has complied with the required health follow-up. When the applicant returns to the civil surgeon’s office, the civil surgeon must:
- Cross out the initial Class A TB classification with a single stroke, and initial and date the change (civil surgeon must indicate on the form that applicant was initially Class A).
- Change the applicant’s status to Class B0, Pulmonary TB. If infectious tuberculosis disease treatment has been prolonged due to multidrug-resistant tuberculosis (MDR TB) or extensively drug-resistant tuberculosis disease (XDR TB), other portions of the medical examination may need to be repeated. When all portions of the examination are current, the civil surgeon can sign the “Civil Surgeon Certification” section of the I-693 form (Part 7. 8.), indicating that the applicant is medically cleared.
- Indicate the following information in the “Remarks” section of the I-693 form (may attach a separate sheet of paper, if needed):
- The drug regimen used (medication names, dosages, number of doses given)
- The date treatment began (month/year)
- The date treatment was completed (month/year)
- The dates (month/year) and results of the most recent sputum culture tests
- After the I-693 is signed, the Civil Surgeon must enter the case into eMedical.
Applicants undergoing infectious tuberculosis disease treatment can petition for a Class A waiver.
In exceptional situations, applicants undergoing tuberculosis disease treatment can petition for a Class A waiver. Form I-601 must be completed by the applicant. These petitions are reviewed by the U.S. Department of Homeland Security (DHS) on an individual basis and considered in situations with extenuating medical circumstances. CDC/DGMH reviews the application and provides an opinion to DHS regarding the case. DHS then has the final authority to approve or deny the waiver request.
All requests for waivers must be accompanied by prior notification and written approval by the U.S.-based physician accepting responsibility for the applicant’s continued care and treatment and the U.S. local and state health department with jurisdiction.
As soon as the civil surgeon is aware that an applicant has applied for a Class A waiver, the civil surgeon must provide the following to CDC so that CDC can review the case and make a recommendation to DHS:
- Summary of case
- All available and pertinent laboratory results
- All chest x-ray images (in a DICOM format)
Applicants who complete treatment for infectious tuberculosis disease do not need a waiver to complete the status adjustment process. Upon successful completion of treatment, they are no longer considered inadmissable.
Glossary of Abbreviations
| Acronym | Full Phrase |
|---|---|
| CDC | U.S. Centers for Disease Control and Prevention |
| Chest x-ray | Chest radiograph |
| DGMH | Division of Global Migration Health |
| DOT | Directly observed therapy |
| DST | Drug susceptibility testing |
| FDA | U.S. Food and Drug Administration |
| HIV | Human immunodeficiency virus |
| IDSA | Infectious Diseases Society of America |
| IGRA | Interferon-gamma release assay |
| LTBI | Latent tuberculosis infection |
| PPD | Purified protein derivative |
| TST | Tuberculin skin test |
Definitions of Selected Terms
Contact – a person who has shared the same enclosed air space (i.e., was exposed) in a household or other enclosed environment for a prolonged period (days or weeks, not minutes or hours) with a person with smear- or culture-positive infectious tuberculosis disease. Contacts exposed in this fashion to persons with smear- or culture-positive infectious tuberculosis disease are at increased risk of infection with M. tuberculosis.
Directly observed therapy (DOT) – adherence-enhancing strategy in which a health-care worker watches a patient swallow each dose of medication in person and documents the dose. Health-care workers providing DOT can include pharmacists, trained community health workers, etc., but cannot include the applicant’s friends or relatives. DOT is the standard of care for all applicants with tuberculosis disease.
Drug susceptibility test (DST) – a laboratory determination to assess whether an M. tuberculosis complex isolate is susceptible or resistant to antituberculosis drugs. The results predict whether a specific drug is likely to be effective in treating tuberculosis disease caused by that isolate.
Extensively drug-resistant tuberculosis disease (XDR TB) – tuberculosis disease caused by M. tuberculosis organisms that are resistant to isoniazid rifampin, a fluoroquinolone, and a second-line injectable (amikacin, capreomycin, and kanamycin) OR isoniazid, rifampin, a fluoroquinolone, and bedaquiline or linezolid.
Extrapulmonary tuberculosis – tuberculosis disease in any part of the body other than the lung parenchyma, pleura, intrathoracic lymph nodes or larynx. The presence of extrapulmonary disease does not exclude pulmonary tuberculosis disease.
Infectious tuberculosis disease – tuberculosis disease of the lung parenchyma, pleura, intrathoracic lymph nodes or larynx. Latent tuberculosis infection and extrapulmonary tuberculosis are not included in this definition of infectious tuberculosis disease.
Interferon gamma release assay (IGRA) – test that measures a component of cell-mediated immunity reactivity to M. tuberculosis in fresh whole blood.
Latent tuberculosis infection (LTBI) – the presence of M. tuberculosis in the body without signs or symptoms, or radiographic or bacteriologic evidence of tuberculosis disease or extrapulmonary tuberculosis.
Laryngeal tuberculosis – tuberculosis disease of the larynx (voice box), a rare form of tuberculosis which is highly infectious.
Multidrug-resistant tuberculosis disease (MDR TB) – tuberculosis disease caused by M. tuberculosis organisms that are resistant to at least isoniazid and rifampin.
M. tuberculosis complex – includes M. tuberculosis, M. bovis, M. africanum, M. microti, M. canetti, M. caprae, M. pinnipedii, and M. orygis.
M. tuberculosis culture – a laboratory test in which the organism is grown from a submitted specimen (e.g., sputum) to determine the presence of M. tuberculosis. In the absence of cross-contamination, a positive culture confirms the diagnosis of tuberculosis disease.
Pleural tuberculosis – tuberculosis disease of the membranes lining the lung, often involving fluid accumulation in the pleural space. Pleural tuberculosis is included in the definition of infectious tuberculosis disease, and is not considered extrapulmonary for the purpose of this document, because parenchymal disease is also often present and may not be apparent on chest radiograph because of compression of affected lung tissue by pleural fluid.
Pulmonary tuberculosis – tuberculosis disease that involves the lung parenchyma and is often infectious (i.e., contagious [determined by sputum smear examination for acid-fast bacilli (AFB) and mycobacterial culture]).
Tuberculosis disease – disease caused by infection with a member of the M. tuberculosis complex that has progressed to causing clinical (manifesting symptoms or signs) or subclinical (early stage in which signs or symptoms are not present, but other indications of disease activity are present) illness.