Lymphatic Filariasis
[Wuchereria bancrofti] [Brugia malayi][Brugia timori]
Causative Agents
The causative agents of lymphatic filariasis (LF) include the mosquito-borne filarial nematodes Wuchereria bancrofti, Brugia malayi, B. timori An estimated 90% of LF cases are caused by W. bancrofti (Bancroftian filariasis).
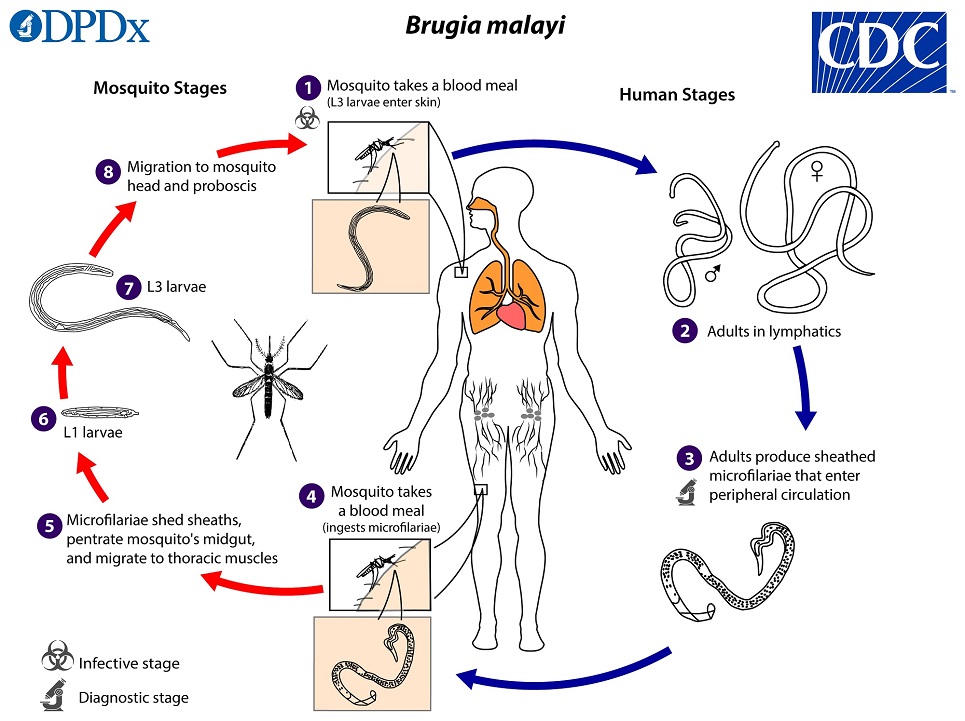
Brugia Malayi Life Cycle
During a blood meal, an infected mosquito (typically Mansonia spp. and Aedes spp.) introduces third-stage filarial larvae onto the skin of the human host, where they penetrate into the bite wound  . They develop into adults that commonly reside in the lymphatics
. They develop into adults that commonly reside in the lymphatics  . The adult worms outwardly resemble those of Wuchereria bancrofti but are smaller. Female worms measure 43 to 55 mm in length by 130 to 170 μm in width, and males measure 13 to 23 mm in length by 70 to 80 μm in width. Adults produce microfilariae, measuring 177 to 230 μm in length and 5 to 7 μm in width, which are sheathed and have nocturnal periodicity (in some regions B. malayi may be sub-periodic, and note that microfilariae are usually not produced in B. pahangi infections). The microfilariae migrate into lymph and enter the blood stream reaching the peripheral blood
. The adult worms outwardly resemble those of Wuchereria bancrofti but are smaller. Female worms measure 43 to 55 mm in length by 130 to 170 μm in width, and males measure 13 to 23 mm in length by 70 to 80 μm in width. Adults produce microfilariae, measuring 177 to 230 μm in length and 5 to 7 μm in width, which are sheathed and have nocturnal periodicity (in some regions B. malayi may be sub-periodic, and note that microfilariae are usually not produced in B. pahangi infections). The microfilariae migrate into lymph and enter the blood stream reaching the peripheral blood  . A mosquito ingests the microfilariae during a blood meal
. A mosquito ingests the microfilariae during a blood meal  . After ingestion, the microfilariae lose their sheaths and work their way through the wall of the proventriculus and cardiac portion of the midgut to reach the thoracic muscles
. After ingestion, the microfilariae lose their sheaths and work their way through the wall of the proventriculus and cardiac portion of the midgut to reach the thoracic muscles  . There the microfilariae develop into first-stage larvae
. There the microfilariae develop into first-stage larvae  and subsequently into third-stage larvae
and subsequently into third-stage larvae  . The third-stage larvae migrate through the hemocoel to the mosquito’s proboscis
. The third-stage larvae migrate through the hemocoel to the mosquito’s proboscis  and can infect another human when the mosquito takes a blood meal
and can infect another human when the mosquito takes a blood meal  .
.
Wuchereria bancrofti Life Cycle
During a blood meal, an infected mosquito introduces third-stage filarial larvae onto the skin of the human host, where they penetrate into the bite wound  . They develop in adults that commonly reside in the lymphatics
. They develop in adults that commonly reside in the lymphatics  . The female worms measure 80 to 100 mm in length and 0.24 to 0.30 mm in diameter, while the males measure about 40 mm by 1 mm. Adults produce microfilariae measuring 244 to 296 μm by 7.5 to 10 μm, which are sheathed and have nocturnal periodicity, except the South Pacific microfilariae which have the absence of marked periodicity. The microfilariae migrate into lymph and blood channels moving actively through lymph and blood
. The female worms measure 80 to 100 mm in length and 0.24 to 0.30 mm in diameter, while the males measure about 40 mm by 1 mm. Adults produce microfilariae measuring 244 to 296 μm by 7.5 to 10 μm, which are sheathed and have nocturnal periodicity, except the South Pacific microfilariae which have the absence of marked periodicity. The microfilariae migrate into lymph and blood channels moving actively through lymph and blood  . A mosquito ingests the microfilariae during a blood meal
. A mosquito ingests the microfilariae during a blood meal  . After ingestion, the microfilariae lose their sheaths and some of them work their way through the wall of the proventriculus and cardiac portion of the mosquito’s midgut and reach the thoracic muscles
. After ingestion, the microfilariae lose their sheaths and some of them work their way through the wall of the proventriculus and cardiac portion of the mosquito’s midgut and reach the thoracic muscles  . There the microfilariae develop into first-stage
. There the microfilariae develop into first-stage  larvae and subsequently into third-stage infective larvae
larvae and subsequently into third-stage infective larvae  . The third-stage infective larvae migrate through the hemocoel to the mosquito’s prosbocis
. The third-stage infective larvae migrate through the hemocoel to the mosquito’s prosbocis  and can infect another human when the mosquito takes a blood meal
and can infect another human when the mosquito takes a blood meal  .
.
Hosts and Vectors
Wuchereria bancrofti, Brugia malayi, and B. timori are considered human parasites as animal reservoirs are of minor epidemiologic importance or absent; felid species and some primates are the primary reservoir hosts of zoonotic B. pahangi.
The typical vector for Brugia spp. filariasis are mosquito species in the genera Mansonia and Aedes. W. bancrofti is transmitted by many different mosquito genera/species, depending on geographical distribution. Among them are Aedes spp., Anopheles spp., Culex spp., Mansonia spp., and Coquillettida juxtamansonia.
Geographic Distribution
W. bancrofti was once widespread in tropical regions globally but control measures have reduced its geographic range. It is currently endemic throughout Sub-Saharan Africa (excluding the southern portion of the continent), Madagascar, several Western Pacific Island nations and territories and parts of the Caribbean. Bancroftian filariasis also occurs sporadically in South America, India, and Southeast Asia.
Brugia spp. associated with LF are more geographically limited and occur only in Southeast Asia. Like W. bancrofti, control measures have reduced the occurrence and endemic range considerably. Brugia timori is restricted to the Lesser Sunda Islands of Indonesia.
Clinical Features
While severe manifestations do not develop in the majority of infections, LF is a potentially highly disfiguring and disabling disease. The most prominent clinical feature is the development of severe lymphedema of the limbs (“elephantiasis”) and occasionally genitalia (hydrocele) due to dysfunction of lymphatic vessels. Affected limbs become grossly swollen; the skin may become thick and pitted, and secondary infection are frequent due to lymphatic dysfunction. Scrotal hydrocele is also seen in some infected males. Lymphangitis, lymphadenopathy, and eosinophilia may accompany infection in the early stages.
A chronic syndrome called “tropical pulmonary eosinophilia” has been associated with W. bancrofti and B. malayi infections, involving eosinophilic pulmonary infiltrate, peripheral hypereosinophilia, wheezing, chest pain, splenomegaly, and bloody sputum. This has most frequently been documented in South and Southeast Asia.
Microfilariae of Wuchereria bancrofti.
The microfilariae of Wuchereria bancrofti are sheathed and measure 240—300 µm in stained blood smears and 275—320 µm in 2% formalin. They have a gently curved body, and a tail that is tapered to a point. The nuclear column (the cells that constitute the body of the microfilaria) is loosely packed; the cells can be visualized individually and do not extend to the tip of the tail. Microfilariae circulate in the blood.





Adults of Wuchereria bancrofti are long and threadlike. The males measure up to 40 mm long and females are 80—100 mm long. Adults are found primarily in lymphatic vessels, less commonly in blood vessels.

Microfilariae of Brugia malayi are sheathed and in stained blood smears measure 175—230 µm. In 2% formalin they are longer, measuring 240—300 µm. The tail is tapered, with a significant gap between the terminal and subterminal nuclei. Microfilaria circulate in the blood.


Microfilaria of Brugia timori are sheathed and measure on average 310 µm in stained blood smears and 340 µm in 2% formalin. Microfilaria of B. timori differ from B. malayi by a having a longer cephalic space, a sheath that does not stain with Giemsa, and a larger number of single-file nuclei towards the tail. Microfilariae circulate in the blood.






Brugia spp. have typical features of filarial nematodes in cross-section. Females reach a maximum diameter of 180 µm; males are smaller (up to 90 µm). The intestine is small and females have two uterine tubes containing developing microfilariae. The musculature is comprised of few coelomyarian cells per quadrant and the cuticle is smooth.





Diagnostic Findings
Microscopy
Lymphatic filariasis is usually identified by the finding of microfilaria in peripheral blood smears (thick or thin) stained with Giemsa or hematoxylin-and-eosin and subsequent morphologic species identification. For increased sensitivity, concentration techniques can be used. These include centrifugation of the blood sample lysed in 2% formalin (Knott’s technique), or filtration through a polycarbonate membrane. Microfilariae of Wuchereria bancrofti and Brugia spp. exhibit a nocturnal periodicity and an accurate diagnosis is best achieved on blood collected at night. W. bancrofti that are sub-periodic may be encountered in some regions of the Pacific Islands, eastern Malaysia, and Vietnam. Sub-periodic B. malayi also occurs in parts of Malaysia. Adults of both species may be identified in biopsy specimens of lymphatic tissue.
Antigen Detection
Antigen detection using an immunoassay for circulating filarial antigens constitutes a useful diagnostic approach because sensitivity for detection of microfilariae can be low and variable. Unlike microfilariae with nocturnal periodicity, filarial antigens can be detected in blood samples collected at any time of day. A rapid format immunochromatographic test has been shown to be a useful and sensitive tool for the detection of Wuchereria bancrofti antigen and is being used widely by lymphatic filariasis elimination programs. Currently, this test is not licensed for use in the United States and cannot be used for patient diagnosis.
Antibody Detection
Serologic enzyme immunoassay tests, including antifilarial IgG1 and IgG4, provide an alternative to microscopic detection of microfilariae for the diagnosis of lymphatic filariasis. Patients with active filarial infection typically have elevated levels of antifilarial IgG4 in the blood and these can be detected using routine assays.
Laboratory Safety
Standard precautions for the processing of blood specimens apply.
Suggested Reading
Crossing the billion. Lymphatic filariasis, onchocerciasis, schistosomiasis, soil-transmitted helminthiases and trachoma: preventive chemotherapy for neglected tropical diseases. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
Tan, L.H., Fong, M.Y., Mahmud, R., Muslim, A., Lau, Y.L. and Kamarulzaman, A., 2011. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitology international, 60 (1), pp.111-113.
Taylor, M.J., Hoerauf, A. and Bockarie, M., 2010. Lymphatic filariasis and onchocerciasis. The Lancet, 376 (9747), pp.1175-1185.
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.