
Evaluating Uptake of Evidence-Based Interventions in 355 Clinics Partnering With the Colorectal Cancer Control Program, 2015–2018
IMPLEMENTATION EVALUATION — Volume 19 — May 19, 2022
Annette E. Maxwell, DrPH1; Amy DeGroff, PhD2; Sarah D. Hohl, PhD3; Krishna P. Sharma, PhD2; Juzhong Sun2; Cam Escoffery, PhD4; Peggy A. Hannon, PhD3 (View author affiliations)
Suggested citation for this article: Maxwell AE, DeGroff A, Hohl SD, Sharma KP, Sun J, Escoffery C, et al. Evaluating Uptake of Evidence-Based Interventions in 355 Clinics Partnering With the Colorectal Cancer Control Program, 2015–2018. Prev Chronic Dis 2022;19:210258. DOI: http://dx.doi.org/10.5888/pcd19.210258.
PEER REVIEWED
What is already known about the topic?
Evidence-based interventions, such as patient and provider reminders, provider assessment and feedback, small media, and patient navigation are effective in increasing colorectal cancer screening.
What is added by this report?
Our evaluation of primary care clinics that participated in the Centers for Disease Control and Prevention’s Colorectal Cancer Control Program showed a high uptake of interventions that can be integrated into electronic medical records but limited uptake of patient navigation and community health workers.
What are the implications for public health practice?
Future research should further explore the use of patient navigation and community health workers in colorectal cancer prevention because these strategies are uniquely suited to reducing cancer disparities.
Abstract
Purpose and Objectives
Colorectal cancer screening rates remain suboptimal in the US. The Colorectal Cancer Control Program (CRCCP) of the Centers for Disease Control and Prevention (CDC) seeks to increase screening in health system clinics through implementation of evidence-based interventions (EBIs) and supporting activities (SAs). This program provided an opportunity to assess the uptake of EBIs and SAs in 355 clinics that participated from 2015 to 2018.
Intervention Approach
The 30 funded awardees of CRCCP partnered with clinics to implement at least 2 of 4 EBIs that CDC prioritized (patient reminders, provider reminders, reducing structural barriers, provider assessment and feedback) and 4 optional strategies that CDC identified as SAs (small media, professional development and provider education, patient navigation, and community health workers).
Evaluation Methods
Clinics completed 3 annual surveys to report uptake, implementation, and integration and perceived sustainability of the priority EBIs and SAs.
Results
In our sample of 355 clinics, uptake of 4 EBIs and 2 SAs significantly increased over time. By year 3, 82% of clinics implemented patient reminder systems, 88% implemented provider reminder systems, 82% implemented provider assessment and feedback, 76% implemented activities to reduce structural barriers, 51% implemented provider education, and 84% used small media. Most clinics that implemented these strategies (>90%) considered them fully integrated into the health system or clinic operations and sustainable by year 3. Fewer clinics used patient navigation (30%) and community health workers (19%), with no increase over the years of the study.
Implications for Public Health
Clinics participating in the CRCCP reported high uptake and perceived sustainability of EBIs that can be integrated into electronic medical record systems but limited uptake of patient navigation and community health workers, which are uniquely suited to reduce cancer disparities. Future research should determine how to promote uptake and assess cost-effectiveness of CRCCP interventions.
Introduction
Screening reduces deaths related to colorectal cancer (CRC), the second-leading cause of cancer death in the US (1). However, despite recommendation by the US Preventive Services Task Force, CRC screening rates remain suboptimal (66% in 2018) (2); rates among uninsured and low-income populations are even lower. For example, in 2018, only about 30% of people who were uninsured and fewer than 50% of individuals who received care at Federally Qualified Health Centers, government-supported safety net clinics, were up to date with CRC screening (2,3).
The Community Preventive Services Task Force oversees rigorous, systematic reviews of the scientific literature to identify prevention strategies with evidence of effectiveness. On the basis of these reviews, the Task Force recommends the following evidence-based interventions (EBIs) to increase CRC screening: patient reminders, provider reminders, reducing structural barriers, provider assessment and feedback, small media, one-on-one education, and community health workers, including patient navigators (4) (Table 1). Few studies have evaluated the uptake and sustainability of EBIs in a large sample of health care clinics (5,6). Such data are needed to understand how these interventions affect population health, as well as how best to increase the scale of effective interventions. Scalability is defined as the ability of an efficacious health intervention to be expanded under real-world conditions to reach a large proportion of the eligible population (7).
In 2015, the Centers for Disease Control and Prevention (CDC) funded the Colorectal Cancer Control Program (CRCCP) with the goal of increasing CRC screening. Thirty awardees were required to partner with primary care clinics that serve high-need populations to implement EBIs to increase CRC screening. On the basis of recommendations from the Task Force, CDC named 4 EBIs as priority for implementation (patient reminders, provider reminders, reducing structural barriers, provider assessment and feedback). These 4 priority EBIs can be implemented at the health system level to change screening rates. CDC deemed the 4 other EBIs that focus on the individual level (small media, one-on-one education, community health workers, patient navigators) as optional supporting activities (SAs). Awardees could implement both EBIs and SAs.
Purpose and Objectives
The primary purpose of this analysis was to determine the uptake and sustainability of EBIs and SAs in clinics participating in the CRCCP program over 3 years, from 2015 to 2018. We define uptake as the initial decision to employ an EBI or SA in a clinic setting (also called adoption), while sustainability indicates integration of an EBI or SA into a clinic’s ongoing operation (8). With regard to SAs, we were especially interested in the uptake of patient navigation by these clinics because most clinics in the CDC program are Federally Qualified Health Centers that provide care to underserved and under-resourced populations that experience health disparities, and patient navigation is a strategy intended to reduce disparities by helping patients overcome barriers to health care (9). Patient navigation is well accepted in these populations (10–13) and can be integrated into existing roles in clinical settings (14–16). The Task Force recently added patient navigation, conducted by patient navigators or community health workers, to their list of recommended interventions to promote CRC screening because it increases CRC screening rates (11,17). CDC defines patient navigation for CRC screening as individualized assistance offered to patients to help address barriers and facilitate timely access to quality screening and follow-up, as well as initiation of treatment services for people diagnosed with cancer. Patient navigation includes assessment of patient barriers, patient education, resolution of barriers, and patient tracking and follow-up. Patient navigation can be provided by health care providers (eg, nurses) or lay workers (eg, community health workers) (18).
Intervention Approach
The CRCCP uses a 5-year funding cycle, and our analysis focused on the 2015 through 2020 cycle. The 30 funded awardees partnered with clinics and provided technical assistance and resources to implement Task Force–recommended EBIs. For this screening program, awardees were required to implement at least 2 of the CDC-prioritized EBIs, as well as SAs; however, awardees were not allowed to use SAs as stand-alone activities. In addition, small media, in particular, had to be paired with 1 of the 4 EBIs (eg, a mailed patient reminder could include a small media material). The screening program is based on several tenets, including integrating public health and primary care, focusing on populations with a high prevalence of disease, implementing sustainable health system changes, and using evidence-based approaches to maximize limited public health dollars (18,19). CRCCP provided an opportunity to study the uptake and sustainability of different EBIs and SAs in a large number of health system clinics that provide care to medically underserved patients and to consider their scalability. Previous studies of this program observed that the implementation of its strategies was associated with increased clinic-level screening rates (18,20).
Evaluation Methods
CDC’s Framework for Program Evaluation was applied to design the clinic survey on which this analysis is based (21). Other components of the CRCCP evaluation include an annual survey of awardees (22), cost effectiveness studies (23), case studies, and studies to explore specific components of CRCCP (18,24).
The clinic survey was based on prior surveys (5,25) and was completed by 1 representative per clinic, similar to other studies (25,26). Data collected in the surveys included clinic characteristics such as clinic type and size, EBIs and SAs in place at baseline and annually, use of CDC resources (eg, staff time, funds, materials) toward implementing EBIs and SAs, sustainability of EBIs and SAs, and baseline and annual CRC screening rates (21). Uptake was defined as EBIs and SAs that are in place and operational (in use) in a clinic at the end of the reporting period. Respondents were asked about sustainability using the question, “If in place, do you consider the EBI or SA as fully integrated into health system or clinic operations and sustainable?” “High quality implementation has been achieved and a supporting infrastructure is in place along with any financial support needed to maintain the EBI/SA. The EBI/SA has become an institutionalized component of the health system and/or clinic operation” was provided as an explanation. Respondents were not asked to consider the length of time that the strategy had been implemented in their responses. Definitions for EBIs and SAs that were given to survey respondents are provided (Table 1). Awardees compiled and reported data to CDC from annual clinic surveys for each participating clinic for each of the first 3 years, from 2015 to 2018.
Statistical analysis
The study sample was limited to clinics that enrolled in the first year of CDC’s screening program (2015–2016) and remained in the program for 3 years (N = 355 clinics, 85% of 417 clinics enrolled). We conducted a descriptive analysis to 1) identify the proportion of clinics implementing the 4 priority EBIs and 4 SAs for each year of the study period and 2) assess whether the EBIs and SAs were perceived as integrated and sustainable by the end of the study period. For each EBI and SA, trends in use between baseline and year 3 were analyzed by using the Cochran–Armitage test for trend. Analyses were conducted using SAS software, version 9.4 (SAS Institute Inc).
Results
Clinic characteristics
Most clinics were Federally Qualified Health Centers (73%), and clinic size varied. Some clinics had fewer than 500 patients aged 50 to 75 years (24%), and others had more than 1,500 patients (38%). The number of providers ranged from fewer than 5 providers per clinic (42%) to more than 20 providers (12%). Patient populations ranged from less than 5% uninsured, aged 50 to 75 (29% of clinics) to more than 20% uninsured patients (36% of clinics). Thirty-four percent of all clinics had access to free fecal testing kits. Most clinics used stool-based tests as their primary CRC screening test (56%); 29% referred patients for colonoscopy, and in 13% of clinics, the primary screening test varied by provider (Table 2).
Uptake of strategies to promote CRC screening
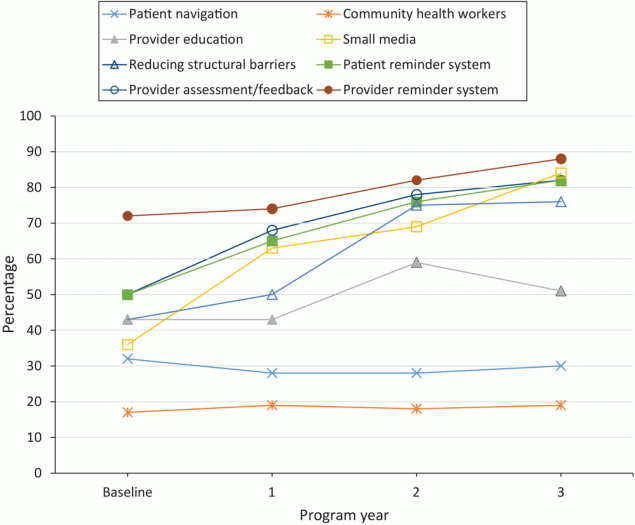
Uptake of strategies to promote CRC screening among clinics varied widely at baseline and throughout the study. At baseline, 50% of clinics used patient reminder systems, 72% implemented provider reminder systems, 50% used provider assessment and feedback, and 43% implemented activities to reduce structural barriers. Significant increases were observed in the uptake of all 4 EBIs in the first 3 years of the program (P < .001 for all 4 EBIs). In year 3, 82% of clinics implemented patient reminder systems, 88% implemented provider reminder systems, and 82% implemented provider assessment and feedback. At baseline, SA use was generally low; 17% of clinics used community health workers, 32% offered patient navigation, 36% used small media, and 43% delivered provider education. Among SAs, professional development and provider education increased significantly, from 43% to 51% (P = .001), and use of small media increased significantly, from 36% to 84% (P < .001) of clinics in year 3 (Figure and Table 3).
![]()
Figure.
Percentage of clinics that partnered with the CDC Colorectal Cancer Control Program using evidence-based interventions to promote colorectal cancer screening, analyzed using the Cochran–Armitage trend test, 2015–2018 (N = 355). [A tabular version of this figure is available.]
A substantial number of clinics implemented or resumed new strategies and discontinued or paused strategies during the study period (Table 3). Overall, the proportion of clinics that changed their EBI use from the prior year ranged from 27% to 36% in the first year. These fluctuations tended to decrease in subsequent years, from 13% to 19% of clinics in year 3. SA implementation fluctuated similarly. Only 28% of clinics had patient navigation in place in the second and third year (after baseline) of the program, but almost the same proportion, 25% to 26% of clinics either newly implemented or discontinued patient navigation in the same program years. In the third year, the proportion of clinics that changed their patient navigation status (either new or discontinued) decreased to 10%.
In the first year of the program, clinics that implemented 2 EBIs (n = 64) also implemented on average 1.4 SAs; those that implemented 3 EBIs (n = 102) implemented an average 1.7 SAs, and those that implemented 4 EBIs (n = 110) implemented an average 2.1 SAs. Concurrent implementation of EBIs and SAs was very similar in all program years. Not all clinics, however, implemented 2 priority EBIs in the first year. The percentage of clinics that implemented fewer than 2 EBIs ranged from 22% in the first year to 11% in the second year and to 4% in the third year.
Integration of strategies to promote CRC screening and sustainability
Among clinics that had EBIs and SAs in place by the end of each year, most considered those EBIs and SAs fully integrated into health systems or clinic operations and sustainable with or without CRCCP resources, especially in years 2 and 3 (Table 4). Sustainability and integration into clinic operations during the 3-year period increased most for activities that largely focused on providers, such as provider reminder systems, an increase of 14 percentage points from 79% in year 1 to 93% in year 3. Similarly, full integration of provider assessment and feedback increased 27 percentage points, from 69% to 96% of clinics; full integration of professional development or provider education increased by 16 percentage points, from 76% to 92% of clinics, followed by full integration of small media for an increase of 11 percentage points from 81% to 92% of clinics. Sustainability and integration into clinic operations did not substantially change with patient navigation (5 percentage point increase from 87% to 92% of clinics) and for community health workers (a 3 percentage point decrease, from 99% to 96%) (Table 4).
Implications for Public Health
To our knowledge, this is one of only a few studies examining the uptake of evidence-based interventions to promote CRC screening in a large sample of clinics in 30 states. A 2012 study of 44 Federally Qualified Health Centers in 4 Midwestern states found that 41% of clinics had no CRC screening tracking system, although 79% reported using electronic health records (25). A 2016 cross-sectional survey of 56 Federally Qualified Health Centers in 7 states found that 73% of them implemented patient reminder systems, 77% implemented provider reminder systems, and 82% implemented provider assessment and feedback. The same study found that fewer clinics used patient navigators (50%) and small media (62%) (26). Our study builds on previous research in 3 ways: 1) by corroborating results regarding the implementation of these strategies, 2) by adding information on the uptake of 8 different EBIs and SAs, and 3) by assessing these strategies, their changes in implementation, and their sustainability and integration over a 3-year period in a large sample of clinics.
Overall, we observed significant uptake of 4 priority EBIs and 2 SAs, suggesting that the CRCCP contributed to increasing implementation of these strategies in the participating clinics. Our data suggest that for all strategies experimentation took place in early years of the program until clinics settled on strategies that worked for their particular contexts. In addition, many clinics required more than a year to implement at least 2 priority EBIs. Clinics that implemented fewer EBIs also tended to implement fewer SAs, and vice versa.
The strong and consistent uptake of priority EBIs by CRCCP clinics may exist, in part, because CDC requires that clinics implement 2 of the 4 priority EBIs, awardees provide technical assistance and implementation support to clinics, and for some clinics, financial support is provided by awardees. Another explanation is that some EBIs and SAs can be integrated into clinical practice through clinics’ electronic health records systems. For example, by using data from electronic health records, patient reminder letters can be generated and personalized with each patient’s name and address, preferred language, the name of the patient’s primary care provider, and their history of CRC screening (eg, type and time of most recent test). Although it takes resources to program electronic health records and to set up these strategies initially, clinic health information technology and automated calling and texting systems can support implementation (27,28). Whether clinics can maintain these interventions solely with their own resources after CRCCP technical and financial support has ended remains to be seen.
Implementation of patient navigation and use of community health workers, on the other hand, was much lower than the priority EBIs and remained low over time. CDC’s focus on the 4 priority EBIs and on sustainability could be the reason for low implementation of these activities. Patient navigation is resource intensive, requiring ongoing funding and dedicated staff. In one study, trained nurse navigators spent an average of 124 minutes per patient to deliver a 6-step protocol by telephone to navigate patients for colonoscopy (29). In addition, the costs of patient navigation can be substantial. An economic analysis of detailed activity-based cost information that was systematically collected in a subset of CRCCP clinics showed costs per person screened ranging from $24 to $40 in 14 clinics that implemented multicomponent interventions that included patient reminders and provider assessment and feedback. The cost per person screened was $134, however, in a clinic that included patient incentives and patient navigation in addition to patient reminders (30). In contrast, some studies have reported that patient navigation resulted in cost savings, especially for endoscopic facilities (31,32). A study that compared patients who were navigated to a screening colonoscopy with non-navigated patients at 1 endoscopy clinic found that navigated patients were significantly more likely to complete colonoscopy and to have adequate bowel preparation. The group of navigated patients also had significantly fewer no-shows and cancellations than the group of non-navigated patients (33). A business case has been made to support patient navigation in some clinical systems that led to increased revenues because of increased patient retention, physician loyalty, reduction in emergency department visits, hospitalizations, and reduced burdens on oncology providers (34). Some of these benefits of patient navigation, however, might not be immediate and might not be assessed. If they are assessed, benefits might not be attributed to patient navigation. As most CRCCP clinics are Federally Qualified Health Centers that might not realize many of the potential economic benefits because patients often go to endoscopy providers. CDC is planning to conduct comparative effectiveness studies to further elucidate cost-effectiveness and other barriers to implement patient navigation. For now, reimbursement through health insurers might be required to increase the scalability of this strategy in primary care settings serving populations most likely to benefit from patient navigation.
Many of the strategies that clinics are implementing, including provider reminders, patient reminders, provider assessment and feedback, and small media, have the potential to promote CRC screening for all patients, and they were associated with screening rate improvements in the first year of the CRCCP (18). With the CRCCP focus on Federally Qualified Health Centers that serve populations with high disease burden, strategies also have the potential to reduce cancer disparities. Patient navigation in particular can focus on patients who have substantial barriers to CRC screening and the least access to care (9,35). This intervention strategy, therefore, is uniquely suited to reduce cancer disparities. Cancer disparities reduction was demonstrated in a statewide CRC screening program in Delaware, population 982,895: 23% Black residents and 69% White residents. The Delaware program included financial coverage for CRC screening, treatment, and patient navigation by nurse coordinators. Statewide CRC screening rates increased from 48% among Black residents and from 58% among White residents in 2001 to 74% in both groups in 2009, and the program resulted in reduced disparities in CRC incidence and mortality (36). Future program evaluations could take a population health equity approach (36,37) by examining patient data of CRCCP clinics to determine if program strategies reduced disparities in CRC screening, stage at diagnosis, incidence, mortality, and which specific strategies contributed to the reduction in CRC disparities. Another set of analyses could examine trends in cancer disparities in the catchment areas of participating clinics during the implementation of CRCCP. An analysis that takes a population health equity approach would add a new perspective to the CRCCP program evaluation and provide crucial information on the value of all program strategies, including EBIs and patient navigation, in reducing CRC disparities. Further research is needed to gain a better understanding of the reasons clinics decide to implement some strategies over others and reasons other strategies are discontinued. Data from these analyses could guide future initiatives to increase CRC screening at a population level.
Our study included a data set with a limited number of variables and did not assess theoretical constructs, such as those of the Consolidated Framework for Implementation Research that might explain uptake and sustainability. Furthermore, many clinics could not provide data on the racial and ethnic characteristics of patients, and we were not able to examine if those characteristics were related to the uptake of intervention strategies, particularly patient navigation. Future studies should assess theoretical constructs that are relevant for implementation to illuminate the determinants of implementation and sustainability (38). We also did not have information about the quality of EBI and SA implementation, which likely varies considerably across clinics. Although respondents were encouraged to consult with their team, surveys were completed by one person per clinic who might not have had complete information. Responses may be influenced by respondent role in the clinic (eg, CRC champion versus a quality improvement specialist) and might also suffer from social desirability bias. Respondents were instructed to not report reducing structural barriers as a patient navigation activity, but it is possible that some respondents conflated these 2 strategies, because patient navigators often conduct work related to reducing structural barriers. Finally, CDC’s mandate that clinics implement at least 2 priority EBIs could have dictated to some extent the selection of strategies (priority EBIs versus optional SAs).
Our analysis focused on the uptake of 8 different strategies, all recommended by the Community Preventive Services Task Force, in a large number of clinics. Those clinics chose which EBIs and SAs to implement in the context of seeking to meet CRCCP program requirements. Primary care clinics participating in the CRCCP significantly increased implementation of 4 priority EBIs (patient reminder systems, provider reminder systems, provider assessment and feedback, and activities to reduce structural barriers) and 2 optional SAs (provider education and small media) to increase CRC screening over the first 3 program years. Uptake may be facilitated through technical and financial support provided by CRCCP awardees and integration of these strategies into clinic electronic health records systems. Implementation of patient navigation and community health workers remained flat over time, likely due, in part, to the need for ongoing funding for staff. Although use of patient navigation and community health workers may be effective strategies for reaching a clinic’s most underserved patients, additional support or encouragement may be required for clinics to add these services.
Acknowledgments
This work was supported by a contract from the National Association of Chronic Disease Directors with the University of Washington. Additional support was provided by CDC and the National Cancer Institute through the Cancer Prevention and Control Research Network, a network within CDC’s Prevention Research Centers program at Emory University, U48DP006377, and the University of Washington, U48DP005013 and U48DP006398. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC. Data are not publicly available. Drs Maxwell, DeGroff, Hohl, Sharma, Escoffery, and Hannon developed the concept for the manuscript. Mr Sun and Dr Sharma performed the analysis. Dr Maxwell wrote the initial draft. All authors discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript. The authors declare that they have no competing interests. No copyrighted materials or tools were used in this research.
Author Information
Corresponding Author: Annette E. Maxwell, DrPH, Department of Health Policy and Management, University of California, Los Angeles, 650 Charles Young Dr South, A2-125 CHS, Box 956900, Los Angeles, CA 90095-6900. Telephone: (310) 794-9282. E-mail: amaxwell@ucla.edu.
Author Affiliations: 1University of California Los Angeles, Los Angeles, California. 2Centers for Disease Control and Prevention, Atlanta, Georgia. 3University of Washington, Seattle, Washington. 4Emory University, Atlanta, Georgia.
References
- Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. ; US Preventive Services Task Force. Screening for colorectal cancer. JAMA 2021;325(19):1965–77. CrossRef PubMed
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70(3):145–64. CrossRef PubMed
- Muthukrishnan M, Arnold LD, James AS. Patients’ self-reported barriers to colon cancer screening in federally qualified health center settings. Prev Med Rep 2019;15:100896. CrossRef PubMed
- The Community Preventive Services Task Force. The Community Guide. 2016. https://www.thecommunityguide.org/sites/default/files/assets/Cancer-Screening-Multicomponent-Colorectal.pdf. Accessed July 9, 2019.
- Yabroff KR, Zapka J, Klabunde CN, Yuan G, Buckman DW, Haggstrom D, et al. Systems strategies to support cancer screening in U.S. primary care practice. Cancer Epidemiol Biomarkers Prev 2011;20(12):2471–9. CrossRef PubMed
- Wheeler SB, Leeman J, Hassmiller Lich K, Tangka FKL, Davis MM, Richardson LC. Data-powered participatory decision making: leveraging systems thinking and simulation to guide selection and implementation of evidence-based colorectal cancer screening interventions. Cancer J . 2018;24(3):136–43. </jrn CrossRef
- Milat AJ, King L, Bauman AE, Redman S. The concept of scalability: increasing the scale and potential adoption of health promotion interventions into policy and practice. Health Promot Int 2013;28(3):285–98. CrossRef PubMed
- Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38(2):65–76. CrossRef
- Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev 2012;21(10):1614–7. CrossRef PubMed
- Jandorf L, Braschi C, Ernstoff E, Wong CR, Thelemaque L, Winkel G, et al. Culturally targeted patient navigation for increasing African Americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2013;22(9):1577–87. CrossRef PubMed
- Bernardo BM, Zhang X, Beverly Hery CM, Meadows RJ, Paskett ED. The efficacy and cost-effectiveness of patient navigation programs across the cancer continuum: a systematic review. Cancer 2019;125(16): 32147–61. CrossRef PubMed
- Hou SI, Roberson K. A systematic review on US-based community health navigator (CHN) interventions for cancer screening promotion — comparing community- versus clinic-based navigator models. J Cancer Educ 2015;30(1):173–86. CrossRef PubMed
- Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med 2018;178(12):1645–58. CrossRef
- Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer 2011;117(15 Suppl):3539–42.
- Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin . 2011;61(4):237–49. CrossRef
- Rohan EA, Slotman B, DeGroff A, Morrissey KG, Murillo J, Schroy P. Refining the patient navigation role in a colorectal cancer screening program: results from an intervention study. J Natl Compr Canc Netw . 2016;14(11):1371–8. CrossRef
- Sunny A, Rustveld L. The role of patient navigation on colorectal cancer screening completion and education: a review of the literature. J Cancer Educ 2018;33(2):251–9. CrossRef PubMed
- DeGroff A, Sharma K, Satsangi A, Kenney K, Joseph D, Ross K, et al. Increasing colorectal cancer screening in health care systems using evidence-based interventions. Prev Chronic Dis 2018;15: 180029. CrossRef PubMed
- Frieden TR. Six components necessary for effective public health program implementation. Am J Public Health . 2014;104(1):17–22. CrossRef
- Sharma KP, DeGroff A, Maxwell AE, Cole AM, Escoffery NC, Hannon PA. Evidence-based interventions and colorectal cancer screening rates: the colorectal cancer screening program, 2015–2017. Am J Prev Med 2021;61(3):402–9. CrossRef PubMed
- Satsangi A, DeGroff A. Planning a national-level data collection protocol to measure outcomes for the Colorectal Cancer Control Program. J Ga Public Health Assoc 2016;6(5 Suppl):292–7. CrossRef PubMed
- Hannon PA, Maxwell AE, Escoffery C, Vu T, Kohn MJ, Gressard L, et al. Adoption and implementation of evidence-based colorectal cancer screening interventions among cancer control program grantees, 2009–2015. Prev Chronic Dis . 2019;16:180682. CrossRef
- Tangka FKL, Subramanian S, DeGroff AS, Wong FL, Richardson LC. Identifying optimal approaches to implement colorectal cancer screening through participation in a learning laboratory. Cancer 2018;124(21):4118–20. CrossRef PubMed
- Barrington WE, DeGroff A, Melillo S, Vu T, Cole A, Escoffery C, et al. Patient navigator reported patient barriers and delivered activities in two large federally funded cancer screening programs. Prev Med 2019;129:105858. CrossRef PubMed
- Daly JM, Levy BT, Moss CA, Bay CP. System strategies for colorectal cancer screening at federally qualified health centers. Am J Public Health 2015;105(1):212–9. CrossRef PubMed
- Adams SA, Rohweder CL, Leeman J, Friedman DB, Gizlice Z, Vanderpool RC, et al. Use of evidence-based interventions and implementation strategies to increase colorectal cancer screening in federally qualified health centers. J Community Health 2018;43(6):1044–52. CrossRef PubMed
- Davis MM, Freeman M, Shannon J, Coronado GD, Stange KC, Guise JM, et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States — how, what and when? BMC Cancer .2018;18(1):40–135. CrossRef
- Lewkowicz D, Wohlbrandt A, Boettinger E. Economic impact of clinical decision support interventions based on electronic health records. BMC Health Serv Res 2020;20(1):871–904. CrossRef PubMed
- DeGroff A, Gressard L, Glover-Kudon R, Rice K, Tharpe FS, Escoffery C, et al. Assessing the implementation of a patient navigation intervention for colonoscopy screening. BMC Health Serv Res 2019;19(1):803. CrossRef PubMed
- Subramanian S, Tangka FKL, Hoover S. Role of an implementation economics analysis in providing the evidence base for increasing colorectal cancer screening. Prev Chronic Dis 2020;17:190407. CrossRef PubMed
- Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority individuals. Cancer 2015;121(7):1088–97. CrossRef PubMed
- Rice K, Sharma K, Li C, Butterly L, Gersten J, DeGroff A. Cost-effectiveness of a patient navigation intervention to increase colonoscopy screening among low-income adults in New Hampshire. Cancer 2019;125(4):601–9. CrossRef PubMed
- Rice K, Gressard L, DeGroff A, Gersten J, Robie J, Leadbetter S, et al. Increasing colonoscopy screening in disparate populations: Results from an evaluation of patient navigation in the New Hampshire Colorectal Cancer Screening Program. Cancer 2017;123(17):3356–66. CrossRef PubMed
- Kline RM, Rocque GB, Rohan EA, Blackley KA, Cantril CA, Pratt-Chapman ML, et al. Patient navigation in cancer: the business case to support clinical needs. J Oncol Pract 2019;15(11):585–90. CrossRef PubMed
- Freund KM, Battaglia TA, Calhoun E, Darnell JS, Dudley DJ, Fiscella K, et al. Writing Group of the Patient Navigation Research Program. Impact of patient navigation on timely cancer care: the Patient Navigation Research Program. J Natl Cancer Inst 2014;106(6):dju115. . CrossRef
- Grubbs SS, Polite BN, , Carney J Jr., Bowser W, Rogers J, Katurakes N, et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol 2013;31(16):1928–30. CrossRef
- American Association for Cancer Research. Cancer Disparities Progress Report. 2020. Accessed February 21, 2022. http://www.CancerDisparitiesProgressReport.org.
- Kim K, Polite B, Hedeker D, Liebovitz D, Randal F, Jayaprakash M, et al. Implementing a multilevel intervention to accelerate colorectal cancer screening and follow-up in federally qualified health centers using a stepped wedge design: a study protocol. Implement Sci 2020;15(1):96. CrossRef
Tables
| Evidence-based interventions | Definitions provided to participants |
|---|---|
| Patient reminder system | System to remind patients when they are due for screening that is in written form (letter, postcard, email) or by telephone voice messages (including automated messages). |
| Provider reminder system | System to inform providers that a patient is due (or overdue) for screening. Reminders can be provided in different ways, such as in patient charts or by email. |
| Provider assessment and feedback | System to both evaluate provider performance in delivering or offering screening to clients (assessment) and present providers with information about their performance in providing screening services (feedback). |
| Reducing structural barriers | Clinic has assessed structural barriers to colorectal cancer screening and has addressed barriers through 1 or more interventions. Structural barriers are noneconomic burdens or obstacles that make it difficult for people to access cancer screening. Reducing structural barriers does not include patient navigation or community health workers. |
| Supporting activities | |
| Small media | Materials used to inform and motivate people to be screened for cancer, including videos and printed materials, letters, brochures, and newsletters. |
| Professional development and provider education | Activities may include distribution of provider education materials, including screening guidelines and recommendations, or continuing medical education opportunities. |
| Community health workers | Lay health educators with a deep understanding of the community who are often from the community being served. Community health workers work in community settings, in collaboration with a health promotion program, clinic, or hospital, to educate people about cancer screening, promote cancer screening, and provide peer support to people referred to cancer screening. |
| Patient navigation | Patient navigators typically assist clients in overcoming individual barriers to cancer screening. Patient navigation includes assessment of client barriers, client education and support, resolution of client barriers, client tracking, and follow-up. Patient navigation should involve multiple contacts with a client. |
Abbreviation: CDC, Centers for Disease Control and Prevention.
| Characteristics | N (%) |
|---|---|
| Federally Qualified Health Center or community health center | 258 (72.7) |
| Health system–owned or hospital-owned | 49 (13.8) |
| Health department, tribal health center, or other | 32 (9.0) |
| Private or physician owned | 16 (4.5) |
| Number of clinic patients aged 50–75 y | |
| <500 | 85 (23.9) |
| 500–1,500 | 137 (38.6) |
| >1,500 | 133 (37.5) |
| Number of primary care providers | |
| <5 | 150 (42.3) |
| 5–20 | 159 (44.8) |
| >20 | 44 (12.4) |
| Missing | 2 (0.5) |
| Percentage of uninsured patients aged 50–75 y | |
| <5 | 104 (29.3) |
| 5–20 | 94 (26.5) |
| >20 | 129 (36.3) |
| Missing | 28 (7.9) |
| Access to free fecal testing kits | |
| Yes | 121 (34.1) |
| No | 209 (58.9) |
| Unknown | 25 (7.0) |
| Type of primary colorectal cancer screening tests | |
| Stool-based tests | 197 (55.5) |
| Colonoscopy referral | 103 (29.0) |
| Varies by provider | 47 (13.2) |
| Unknown | 8 (2.3) |
| Evidence-based intervention type | Program year | Clinics that changed evidence-based interventions use from prior yeara, N (%) | Evidence-based interventions in placeb | |
|---|---|---|---|---|
| N (%) | P value for trendc | |||
| Patient reminder system | Baseline | NR | 177 (50) | <.001 |
| Year 1 | 128 (36) | 231 (65) | ||
| Year 2 | 86 (24) | 271 (76) | ||
| Year 3 | 45 (13) | 290 (82) | ||
| Provider reminder system | Baseline | NR | 254 (72) | <.001 |
| Year 1 | 96 (27) | 262 (74) | ||
| Year 2 | 54 (15) | 290 (82) | ||
| Year 3 | 45 (13) | 311 (88) | ||
| Provider assessment and feedback | Baseline | NR | 178 (50) | <.001 |
| Year 1 | 129 (36) | 243 (68) | ||
| Year 2 | 61 (17) | 276 (78) | ||
| Year 3 | 66 (19) | 290 (82) | ||
| Reducing structural barrier activities | Baseline | NR | 153 (43) | <.001 |
| Year 1 | 121 (34) | 176 (50) | ||
| Year 2 | 129 (36) | 265 (75) | ||
| Year 3 | 56 (16) | 269 (76) | ||
| Patient navigation | Baseline | NR | 114 (32) | .53 |
| Year 1 | 91 (26) | 101 (28) | ||
| Year 2 | 88 (25) | 101 (28) | ||
| Year 3 | 35 (10) | 106 (30) | ||
| Community health workers | Baseline | NR | 60 (17) | .52 |
| Year 1 | 45 (13) | 69 (19) | ||
| Year 2 | 14 (4) | 63 (18) | ||
| Year 3 | 12 (3) | 69 (19) | ||
| Professional development and provider education | Baseline | NR | 152 (43) | <.001 |
| Year 1 | 153 (43) | 151 (43) | ||
| Year 2 | 90 (25) | 211 (59) | ||
| Year 3 | 83 (23) | 182 (51) | ||
| Small media | Baseline | NR | 127 (36) | <.001 |
| Year 1 | 160 (45) | 225 (63) | ||
| Year 2 | 65 (18) | 246 (69) | ||
| Year 3 | 73 (21) | 297 (84) | ||
Abbreviations: CDC, Centers for Disease Control and Prevention; NR, not reported.
a Clinics that implemented or resumed evidence-based interventions and support activities that were not in place in the prior year or that paused or discontinued those interventions and activities that were in place in the prior year.
b Indicates evidence-based interventions and support activities are in place and operational (in use) in clinics at the end-of-program year, regardless of the quality, reach, or current level of functionality.
c Two-sided P value, Cochran–Armitage trend test.
| Intervention | Clinics that have specific EBI/SA in placea by end of program year, N (%) | Fully integrated EBI/SA in placeb | ||
|---|---|---|---|---|
| Yes, with or without CRCCP resources % | No, % | Unknown or missing, % | ||
| Priority EBIs | ||||
| Patient reminder systems | ||||
| Year 1 | 231 (65) | 84 | 13 | 3 |
| Year 2 | 271 (76) | 95 | 3 | 2 |
| Year 3 | 290 (82) | 93 | 3 | 4 |
| Provider reminder systems | ||||
| Year 1 | 262 (74) | 79 | 13 | 8 |
| Year 2 | 290 (82) | 94 | 4 | 2 |
| Year 3 | 311 (88) | 93 | 3 | 4 |
| Provider assessment and feedback | ||||
| Year 1 | 243 (68) | 69 | 17 | 14 |
| Year 2 | 276 (78) | 93 | 3 | 4 |
| Year 3 | 290 (82) | 96 | 1 | 3 |
| Activities to reduce structural barriers | ||||
| Year 1 | 176 (50) | 91 | 2 | 7 |
| Year 2 | 265 (75) | 97 | 0 | 3 |
| Year 3 | 269 (76) | 98 | 1 | 1 |
| Supporting activities | ||||
| Patient navigation | ||||
| Year 1 | 101 (28) | 87 | 7 | 6 |
| Year 2 | 101 (28) | 93 | 0 | 7 |
| Year 3 | 106 (30) | 92 | 3 | 5 |
| Community health workers | ||||
| Year 1 | 69 (19) | 99 | 0 | 1 |
| Year 2 | 63 (18) | 98 | 0 | 2 |
| Year 3 | 69 (19) | 96 | 0 | 4 |
| Professional development and provider education | ||||
| Year 1 | 151 (43) | 76 | 15 | 9 |
| Year 2 | 211 (59) | 88 | 0 | 12 |
| Year 3 | 182 (51) | 92 | 0 | 8 |
| Small media | ||||
| Year 1 | 225 (63) | 81 | 12 | 7 |
| Year 2 | 246 (69) | 96 | 0 | 4 |
| Year 3 | 297 (84) | 92 | 2 | 6 |
Abbreviations: CRCCP, Colorectal Cancer Control Program; EBI, evidence-based interventions; SA, supporting activities.
a Indicates whether EBI/SA are in place by end of program year, regardless of quality, reach, or level of functionality.
b Indicates whether EBI/SA are fully integrated (institutionalized) by end of program year into the health system or clinic operations with supporting infrastructure and financial support to maintain the EBI/SA.
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions.