The Respiratory Protection Information Trusted Source
Use of NIOSH-approved Respirators
NIOSH is the federal agency responsible for testing and approving respirators used in U.S. workplace settings. When an employer determines that respiratory protection is necessary in a workplace, the Occupational Safety and Health Administration (OSHA) requires the use of NIOSH-approved respirators. Workplaces covered under OSHA must also establish a complete respiratory protection program. OSHA regulation 29 CFR 1910.134 requires a respiratory protection program to include medical evaluations, training, and fit testing.
Respirator Fit Testing
A fit test is performed to confirm the fit of any respirator that forms a tight seal on the wearer’s face. As part of the OSHA regulation 29 CFR 1910.134, fit testing is mandatory for all employees required to wear tight-fitting respirators on the job.
Respirator Safety
A respirator’s effectiveness depends heavily on proper fit and use. Verify you have a NIOSH-approved respirator by checking the Certified Equipment List. For your respirator to provide the protection expected, you must properly don (put on) and doff (take off) it. Each manufacturer provides donning procedures for its respirator models. The NIOSH-Approved Filtering Facepiece Respirators webpage provides manufacturers’ donning procedures for most filtering facepiece respirator models. Always follow the manufacturer’s donning procedures. Additionally, when using a tight-fitting respirator, you must perform a user seal check each time you don the device. Before using a NIOSH-approved respirator, OSHA requires a medical evaluation to determine your ability to wear a respirator.
- Filtering Out Confusion: Frequently Asked Questions about Respiratory Protection, User Seal Check
- NIOSH-Approved Particulate Filtering Facepiece Respirators
- Three Key Factors Required for a Respirator to be Effective
- How to Properly Put On and Take Off a Disposable Respirator
- OSHA Respirator Safety Video
- OSHA Voluntary Use of Respirators Video
- OSHA Respiratory Protection Training Requirements Video
- OSHA Medical Evaluations for Workers Who Use Respirators Video
Buyer Beware
NIOSH NPPTL Director Dr. Maryann D’Alessandro discusses how to identify NIOSH-approved respirators and counterfeits in an interview with UL Standards & Engagement’s Be Safe Buy Real.
Respiratory Protective Device Information
NIOSH provides guidance to users through its Respiratory Protective Device Information notices. These notices include guidance related to topics such as user instructions and degraded product performance. Additionally, NIOSH provides a list of respirator user notices issued by manufacturers to keep users updated on potential risks or conditions that exist with NIOSH-approved respirators. Users and purchasers can also refer to the manufacturer’s website for user notices related to their products.
Counterfeit Respirators/Misrepresentation of NIOSH Approval
Counterfeit respirators did not undergo NIOSH testing and evaluation themselves, but were specifically manufactured to mimic a respirator product that did receive NIOSH-approval. Misrepresentation of the NIOSH approval occurs when a respirator product is falsely marketed and sold as being approved by NIOSH. Both types of products may not be capable of providing appropriate respiratory protection to workers. When reports of counterfeit or misrepresentation are received, NIOSH posts these respirators on the Counterfeit Respirators / Misrepresentation of NIOSH Approval webpage to alert users, purchasers, and manufacturers.
NIOSH is aware of falsely used terms such as “NIOSH-approved” and “NIOSH N95” on respirator packaging. Therefore, employers and respirator users should use NIOSH’s Certified Equipment List or the list of NIOSH-approved filtering facepiece respirators to verify respirators are NIOSH approved. Additionally, it is important to confirm that the product’s model name and NIOSH approval number matches the Certified Equipment List.
Markings and Approval Labels
Some products may look very similar to NIOSH-approved respirators. However, there are two key ways you can identify NIOSH-approved respirators: the NIOSH approval number and the approval label. All NIOSH-approved respirators include an approval number. Since 2008, NIOSH requires approval holders to place the approval number (e.g., TC 84A-XXXX) on the respirator facepiece or straps. Figure 1 shows an example of the correct markings on a NIOSH-approved FFR.
In addition to the approval number, NIOSH-approved respirators have an approval label located on or within the respirator packaging. The NIOSH approval label contains contact information for the manufacturer, cautions and limitations for use, and directions for proper use. Figure 2 shows an example of a NIOSH approval label.
Figure 1: Example of correct markings on NIOSH-approved filtering facepiece respirators.
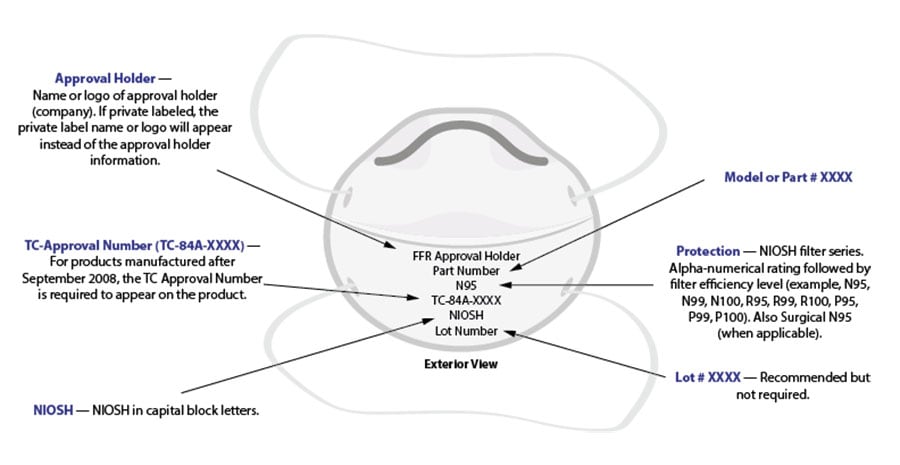
Figure 2: Example of an FFR NIOSH approval label on the respirator packaging

- Respirator Awareness: Your Health May Depend on It
- OSHA Counterfeit and Altered Respirators: The Importance of NIOSH Certification Video
- Counterfeit Respirators/Misrepresentation of NIOSH Approval Webpage
- Misrepresentation of NIOSH Approval Webinar
- Certified Equipment List
- List of NIOSH-Approved Filtering Facepiece Respirators
