NIOSH Conformity Assessment Interpretation Notice
Supersedes: May 18, 2005 Letter to All Respirator Manufacturers
NIOSH CA 2019-1011
January 2019
1 – SUMMARY
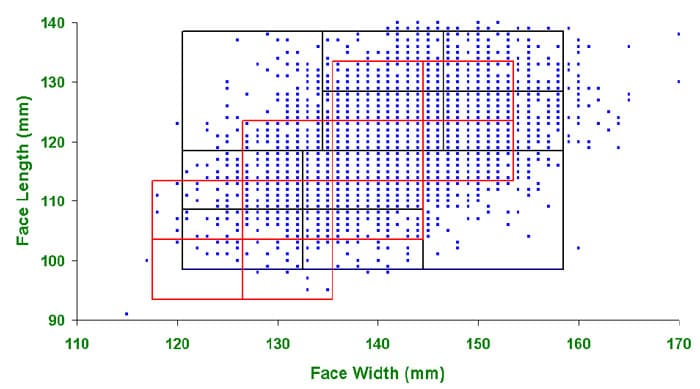
NIOSH is phasing out the use of the Los Alamos National Laboratory (LANL) Panels and updated Standard Testing Procedure (STP) TEB-APR-STP-0005-05a-06, Determination of Qualitative Isoamyl Acetate (IAA) Facepiece Fit, Air-Purifying Respirators, to include use of the NIOSH Bivariate Panel (NIOSH Panel). The NIOSH Panel’s anthropometric limits and individual panel cells (1-10) are shown in Figure 1. The population cell distribution of the NIOSH Panel is given in Table 1, based on an 18- member panel.
Beginning February 1, 2019, NIOSH will implement use of the NIOSH Panel during evaluation of all new approvals of air-purifying respirators (half mask and full-facepiece, excluding non-powered, particulate only air-purifying respirators). These types of approval projects, accepted in the NIOSH Division Electronic Information Management System (DEIMS) on or after February 1, 2019, will be evaluated using the NIOSH Panel and the updated STP. A new approval may include a respirator facepiece configuration previously approved as part of a different respirator approval configuration. Applicants may continue to use the LANL panels to generate pre-submission test data however, NIOSH recommends using the procedure outlined in the updated STP.
Respirators previously approved following testing with LANL Panels continue to be NIOSH-approved respirators. When these approvals are submitted for an extension of approval, and the application requires human subject testing, the respirator will be evaluated using the NIOSH Panel and if a pass is not achieved, the testing will be repeated using the relevant LANL Panel.
After December 31, 2019, when respirators previously approved following testing with LANL Panels are submitted for an extension of approval requiring human subject testing and do not pass the NIOSH Panel, they will not be reevaluated using the LANL Panel.
Certified product audit testing including the use of the anthropometric panels will allow the use of the panel used during the original testing for the approval until December 31, 2019. For example, if the half-facepiece LANL panel was used during the original testing, the half-facepiece LANL panel will be used for certified product audit testing until December 31, 2019.
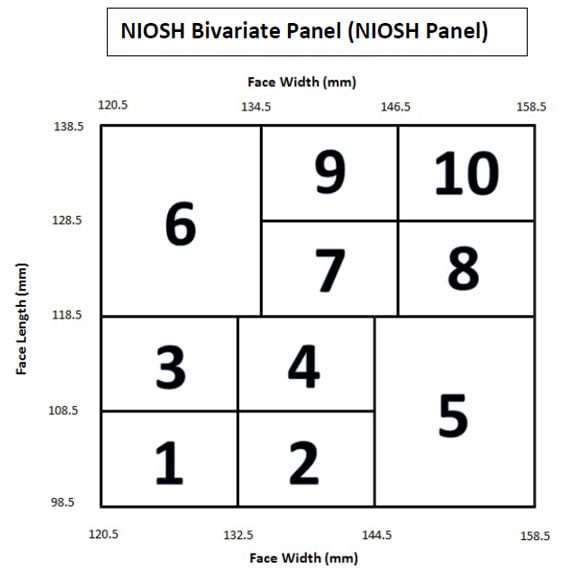
Figure 1. The NIOSH Bivariate Panel (NIOSH Panel) includes ten individual member cells defined by face width and face length (mm)
| NIOSH Panel – cell number | Population distribution (%) | Number of test subjects based on the population distribution |
|---|---|---|
| 1 | 5.5 | 1 |
| 2 | 5.3 | 1 |
| 3 | 10.5 | 2 |
| 4 | 25.0 | 4 |
| 5 | 7.1 | 1 |
| 6 | 5.7 | 1 |
| 7 | 21.3 | 4 |
| 8 | 8.7 | 2 |
| 9 | 5.2 | 1 |
| 10 | 3.5 | 1 |
| Total number of subjects | 18 | |
Table 1: NIOSH Panel cell designations, population distribution, and number of test subjects per cell, based on an 18-member panel
2 – AUTHORITY
42 CFR Part 84, Respiratory Protective Devices
TEB-APR-STP-0005-05a-06, Determination of Qualitative Isoamyl Acetate (IAA) Facepiece Fit, Air-Purifying Respirators
3 – REFERENCES
Approval of Respiratory Protective Devices, 42 CFR, Part 84
NIOSH May 18, 2005 Letter to All Respirator Manufacturers, Air-Purifying Fit Test Subject Selection
Revised TEB-APR-STP-0005-5a-06
Zhuang, Z., Bradmiller, B., Shaffer, R.: New Respirator Fit Test Panels Representing the Current U.S. Civilian Work Force. J. Occup. Environ. Hyg. 4: 647-659 (2007).
Appendix A – BACKGROUND and SUPPLEMENTAL INFORMATION
Prior to Febuary 1, 2019, the NIOSH Respirator Approval Program used human test subjects, identified by the LANL Panels (Figures A1 and A2) to assess fit in accordance with 42 Code of Federal Regulations Part 84. Use of the LANL Panels to assess fit of air-purifying respirators was included in the May 18, 2005 NIOSH Letter to All Respirator Manufacturers, and NIOSH TEB-APR-STP-0005-5a-06. Revision 2.0 (20 March 2008), Determination of Qualitative Isoamyl Acetate (IAA) Facepiece Fit, Air-Purifying Respirators.
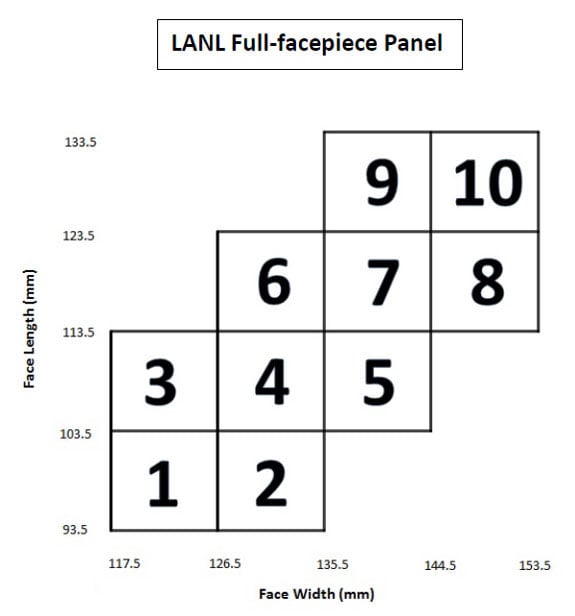
Figure A1. Los Alamos National Laboratory Full-facepiece Panel, with 10 cell boxes identified
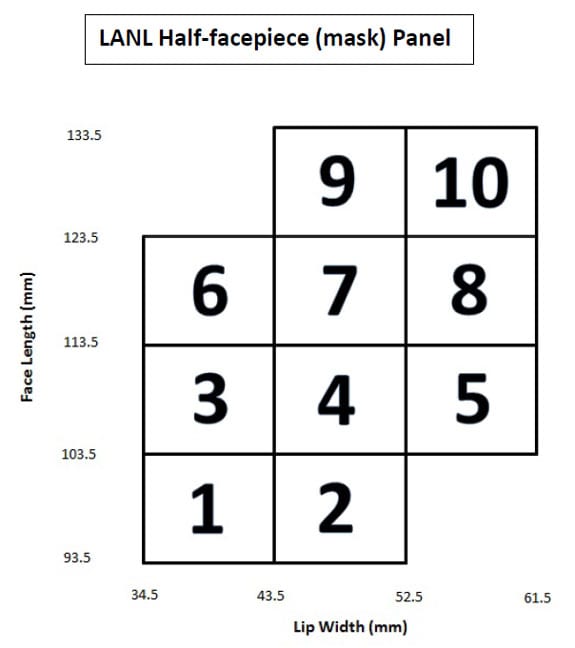
Figure A2. Los Alamos National Laboratory Half-facepiece (mask) Panel, with 10 cell boxes identified