Strongyloidiasis
[Strongyloides stercoralis] [Strongyloides fuelleborni]
Causal Agents
The rhabditid nematode (roundworm) Strongyloides stercoralis is the major causative agent of strongyloidiasis in humans. Rarer human-infecting species of Strongyloides are the zoonotic S. fuelleborni (fülleborni) subsp. fuelleborni and S. fuelleborni subsp. kellyi, for which the only currently known host is humans. Strongyloides spp. are sometimes called “threadworms” (although in some countries this common name refers to Enterobius vermicularis).
Other animal-associated Strongyloides spp., including S. myopotami (nutria), S. procyonis (raccoons), and possibly others, may produce mild short-lived cutaneous infections in human hosts (larva currens, “nutria itch”), but do not cause true strongyloidiasis.
Life Cycle
Strongyloides stercoralis
 , develop into either infective filariform larvae (direct development)
, develop into either infective filariform larvae (direct development)  or free-living adult males and females
or free-living adult males and females  that mate and produce eggs
that mate and produce eggs  , from which rhabditiform larvae hatch
, from which rhabditiform larvae hatch  and eventually become infective filariform (L3) slarvae
and eventually become infective filariform (L3) slarvae  . The filariform larvae penetrate the human host skin to initiate the parasitic cycle (see below)
. The filariform larvae penetrate the human host skin to initiate the parasitic cycle (see below)  . This second generation of filariform larvae cannot mature into free-living adults and must find a new host to continue the life cycle.
. This second generation of filariform larvae cannot mature into free-living adults and must find a new host to continue the life cycle.
Parasitic cycle: Filariform larvae in contaminated soil penetrate human skin when skin contacts soil  , and migrate to the small intestine
, and migrate to the small intestine  . It has been thought that the L3 larvae migrate via the bloodstream and lymphatics to the lungs, where they are eventually coughed up and swallowed. However, L3 larvae appear capable of migrating to the intestine via alternate routes (e.g. through abdominal viscera or connective tissue). In the small intestine, the larvae molt twice and become adult female worms
. It has been thought that the L3 larvae migrate via the bloodstream and lymphatics to the lungs, where they are eventually coughed up and swallowed. However, L3 larvae appear capable of migrating to the intestine via alternate routes (e.g. through abdominal viscera or connective tissue). In the small intestine, the larvae molt twice and become adult female worms  . The females live embedded in the submucosa of the small intestine and produce eggs via parthenogenesis (parasitic males do not exist)
. The females live embedded in the submucosa of the small intestine and produce eggs via parthenogenesis (parasitic males do not exist)  , which yield rhabditiform larvae. The rhabditiform larvae can either be passed in the stool
, which yield rhabditiform larvae. The rhabditiform larvae can either be passed in the stool  (see “Free-living cycle” above), or can cause autoinfection
(see “Free-living cycle” above), or can cause autoinfection  .
.
Rhabditiform larvae in the gut become infective filariform larvae that can penetrate either the intestinal mucosa or the skin of the perianal area, resulting in autoinfection. Once the filariform larvae reinfect the host, they are carried to the lungs, pharynx and small intestine as described above, or disseminate throughout the body. The significance of autoinfection in Strongyloides is that untreated cases can result in persistent infection, even after many decades of residence in a non-endemic area, and may contribute to the development of hyperinfection syndrome.
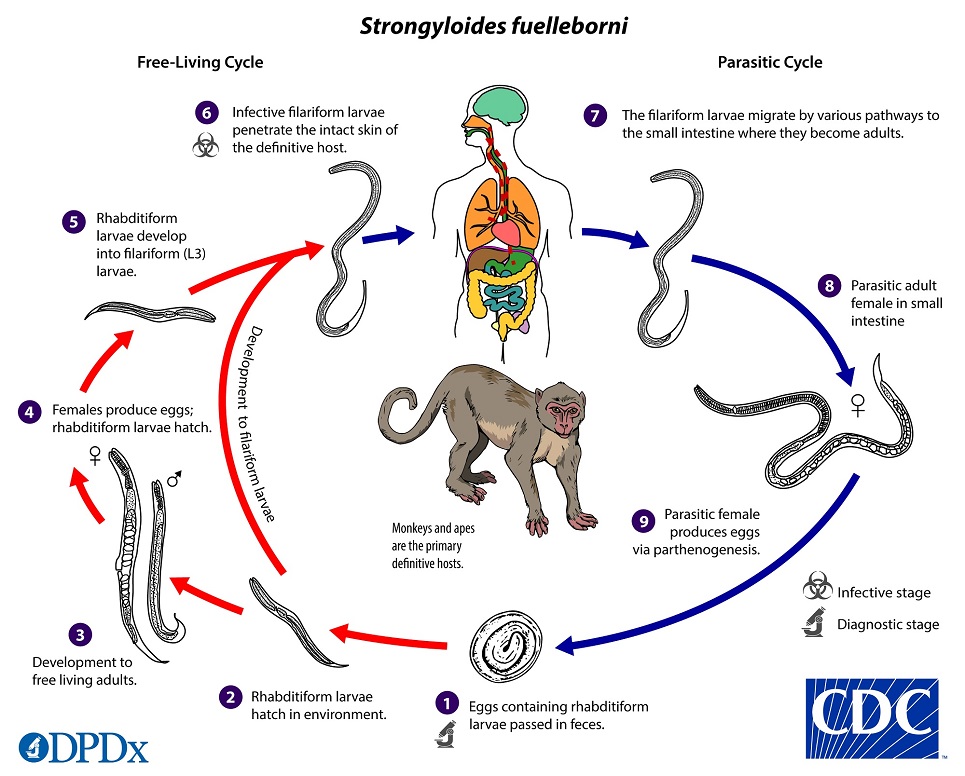
Life Cycle
Strongyloides fuelleborni
Strongyloides fuelleborni follows the same life cycle as S. stercoralis, with the important distinction that eggs (rather than larvae) are passed in the stool  . Eggs hatch shortly after passage into the environment, releasing rhabditiform larvae
. Eggs hatch shortly after passage into the environment, releasing rhabditiform larvae  , that develop to either infective filariform larvae (direct development)
, that develop to either infective filariform larvae (direct development)  or free-living adult males and females
or free-living adult males and females  . The free-living adults mate and produce eggs, from which more rhabditiform larvae hatch
. The free-living adults mate and produce eggs, from which more rhabditiform larvae hatch  and eventually become infective filariform larvae
and eventually become infective filariform larvae  . The filariform larvae penetrate the human host skin to initiate the parasitic cycle
. The filariform larvae penetrate the human host skin to initiate the parasitic cycle  . These larvae migrate via the bloodstream to the lungs, where they are eventually coughed up and swallowed, or reach the intestine via migration through connective tissue or abdominal viscera
. These larvae migrate via the bloodstream to the lungs, where they are eventually coughed up and swallowed, or reach the intestine via migration through connective tissue or abdominal viscera  . In the small intestine, larvae molt twice and become adult female worms. Parasitic females embedded in the submucosa of the small intestine
. In the small intestine, larvae molt twice and become adult female worms. Parasitic females embedded in the submucosa of the small intestine  and produce eggs via parthenogenesis (parasitic males do not exist)
and produce eggs via parthenogenesis (parasitic males do not exist)  .
.
Since eggs do not hatch within the host as with S. stercoralis, autoinfection is believed to be impossible. Transmission of S. fuelleborni subsp. kellyi to infants as a result of breastfeeding has been reported.
Hosts
Strongyloides spp. are generally host-specific, and S. stercoralis is primarily a human parasite. However, patent infections with parasitic females have been detected in other primates (chimpanzees, monkeys, etc.) and domestic dogs. Two genetic populations have been found in domestic dogs, one that appears to only infect dogs and one that may infect both dogs and humans; all human infections have been attributed to this second genetic population. Domestic cats are experimentally susceptible to S. stercoralis infections although it is unknown if they have a role as a natural reservoir.
Strongyloides fuelleborni subsp. fuelleborni is a parasite of Old World apes and monkeys. The only identified host of S. fuelleborni subsp. kellyi is humans.
Geographic Distribution
Strongyloides stercoralis is broadly distributed in tropical and subtropical areas across the globe. Transmission has been reported during summer months in temperate areas. Infections are most common in areas with poor sanitation, rural and remote communities, institutional settings, and among socially marginalized groups.
S. fuelleborni subsp. fuelleborni occurs in non-human primates throughout the Old World. The vast majority of human infections are reported from sub-Saharan Africa. Sporadic cases have been reported from Southeast Asia. S. fuelleborni subsp. kellyi is found in Papua New Guinea, and has not been reported elsewhere thus far.
Clinical Presentation
The initial sign of acute strongyloidiasis, if noticed at all, is a localized pruritic, erythematous rash at the site of skin penetration. Patients may then develop tracheal irritation and a dry cough as the larvae migrate from the lungs up through the trachea. After the larvae are swallowed into the gastrointestinal tract, patients may experience diarrhea, constipation, abdominal pain, and anorexia. Chronic strongyloidiasis is generally asymptomatic, but a variety of gastrointestinal and cutaneous manifestations may occur. Rarely, patients with chronic strongyloidiasis may develop other complications (e.g. arthritis, cardiac arrhythmias, chronic malabsorption, duodenal obstruction, nephrotic syndrome, recurrent asthma). Up to 75% of people with chronic strongyloidiasis have mild peripheral eosinophilia or elevated IgE levels.
Hyperinfection syndrome and disseminated strongyloidiasis are most frequently associated with subclinical infection in patients receiving high-dose corticosteroids. Subsequent impaired host immunity leads to accelerated autoinfection and an overwhelming number of migrating larvae. In chronic strongyloidiasis and in hyperinfection syndrome, the larvae are limited to the GI tract and the lungs, whereas in disseminated strongyloidiasis the larvae invade numerous organs. A variety of systemic, gastrointestinal, pulmonary, and neurologic signs/symptoms have been documented; complications can be severe. Left untreated, the mortality rates of hyperinfection syndrome and disseminated strongyloidiasis can approach 90%.
The subcutaneous migration of filariform larvae in the autoinfective cycle, or “larva currens”, presents as a recurrent serpiginous maculopapular or urticarial rash along the buttocks, perineum, and thighs due to repeated autoinfection. This rash usually advances very rapidly (up to 10 cm/hr).
In infants infected with S. fuelleborni subsp. kellyi, a severe, often fatal, systemic illness involving protein-losing enteropathy has been described, which sometimes presents with peritoneal ascites (“swollen belly syndrome”).
Strongyloides stercoralis first-stage rhabditiform (L1) larvae.
Rhabditiform larvae can be found in stool, as the eggs embryonate and hatch in the mucosa of the small intestine of the host. They may also be found in soil and cultured fecesThe first-stage rhabditiform larvae (L1) of Strongyloides stercoralis are 180—380 µm long, with a short buccal canal, a rhabditoid esophagus (divided into three sections) extending 1/3 of the body length, and a prominent genital primordium. Second-stage rhabditiform larvae (L2) are longer and have a smaller esophagus/intestine ratio.






Strongyloides stercoralis third-stage filariform (L3) larvae.
Infective, third-stage filariform larvae (L3) of Strongyloides stercoralis are up to 600 µm long. The tail is notched and the esophagus to intestine ratio is 1:1, which helps distinguish it from hookworm filariform larvae (which have a short esophagus and pointed tail). Infective L3 larvae are found in soil and invade the human host by direct penetration of the skin. They may be found in respiratory specimens during cases of autoinfection.



Strongyloides stercoralis free-living adults.
Strongyloides stercoralis adult worms may be found in the human host or soil. Parasitic males do not exist; parasitic females are long, slender and measure 2.0—3.0 mm in length. In the environment, rhabditiform larvae may develop into infective filariform (L3) larvae (direct cycle) or free-living male and female adult worms (indirect cycle). Free-living adult males measure up to 0.75 mm long; free-living females measure up to 1.0 mm long.




The following images are from a stool specimen of a child with disseminated S. stercoralis infection. Parasitic females, rhabditiform larvae, and eggs were recovered. This is a rare finding and would not be expected in uncomplicated strongyloidiasis.



Strongyloides stercoralis in tissue.
Adults and larvae of Strongyloides stercoralis in tissue specimens, stained with hematoxylin and eosin (H&E)






Strongyloides fuelleborni eggs
S. fuelleborni produces eggs which are shed in the feces, although hatched rhabditiform larvae may be found if fixation and processing are delayed. They are slightly ovoid (50—60 x 30—40 µm) with a thin, colorless shell, and are passed partially embryonated.



Laboratory Diagnosis
Strongyloidiasis is usually diagnosed by microscopic identification of Strongyloides stercoralis larvae (rhabditiform and occasionally filariform) in the stool, duodenal fluid, and/or biopsy specimens, and possibly sputum in disseminated infections. Examination of serial samples may be necessary, and not always sufficient, because infection burden is often low, larval output is minimal in uncomplicated infections, and microscopic examination of stool has low sensitivity.
S. fuelleborni-strongyloidiasis is diagnosed based on detection of eggs in stool, though hatched rhabditiform larvae may be present if processing is delayed. S. fuelleborni subsp. fuelleborni eggs are passed individually in stool and hatch rapidly; S. fuelleborni subsp. kellyi eggs are often passed within microscopic strings of mucous in the feces. Eggs of both species are smaller than those of hookworms (45—55 x 30—35 µm) have a thin shell and are passed containing larvae which fill the egg and are in at least the cleaved stage of development.
The stool can be examined in wet mounts, either directly or after concentration (note that formalin-ethyl acetate concentration may remove larvae and reduce sensitivity). Larvae are best visualized following recovery by the Baermann funnel sedimentation technique or after culture using Koga agar plate, charcoal culture or the Harada-Mori filter paper technique. The Koga agar plate culture method is recognized in most studies as being the most sensitive coprological method for the detection of Strongyloides larvae.
Aspiration of duodenal fluid or use of the less invasive Entero-test (commonly called a string test) may be useful to detect larvae in patients with negative stool samples. In disseminated strongyloidiasis, filariform larvae may be detected in sputum, bronchial washings or pleural fluid. Patient with rashes may have larvae identified on skin biopsy.
More on: Morphologic comparison with other intestinal parasites.
Antibody Detection
Immunodiagnostic tests for strongyloidiasis are indicated when infection is suspected and the organism is not detected by duodenal aspiration, string tests, or by repeated examinations of stool. Most antibody detection tests employ antigens derived from Strongyloides stercoralis (or from closely-related S. ratti or S. venezuelensis) filariform larvae, although recombinant antigens such as (e.g. NIE, SsIR) are increasingly being used. Although indirect fluorescent antibody (IFA), indirect hemagglutination (IHA) and antigen-linked fluorescent and magnetic bead tests are are available, enzyme immunoassay (EIA) is recommended because of its greater sensitivity. The filariform antigen-based EIA used at CDC has a sensitivity of 96% and a specificity of 98%. The commercial EIA kits that are currently available have comparable specificity but slightly lower sensitivity.
Immunocompromised persons with disseminated strongyloidiasis usually have detectable IgG antibodies despite their immunosuppression, though false negative results can occur. Cross-reactions in patients with filariasis, schistosomiasis, and ascariasis may also occur, depending on the antigen used. Cross-reactivity to sera from S. fuelleborni-infected patients probably occurs, but performance has not been evaluated.
A positive serologic test result warrants continuing efforts to establish a parasitological diagnosis followed by anthelminthic treatment, as positive serology does not differentiate between past and current infection. Serological reversion to antibody negative status is unusual in most strongyloidiasis patients, although antibody levels decrease markedly within 6 months after successful chemotherapy. Thus, serologic monitoring may be useful in the follow-up of treated patients.
Molecular Detection
PCR and LAMP methods may be used to detect strongyloidiasis in fresh, frozen, or non-formalin fixed stool specimens. Sensitivity and specificity vary depending on the reference test used to calculate such characteristics; false negatives and false positives do occur.
Laboratory Safety
Standard precautions for the processing of stool specimens apply. Unfixed stools and/or larval coprocultures should be handled with caution to avoid percutaneous exposure to filariform larvae. Agar plate cultures should be sealed with parafilm or masking tape prior to incubation. Because filariform larvae often migrate to condensation droplets on the lids of agar plate cultures care should be taken when reading cultures or opening culture plates. PPE including gloves, gowns, and eyewear should always be worn when working with samples potentially containing live larvae. Lugol’s iodine (1% povidine iodine; 10,000 ppm) or 70-100% ethanol may be used to kill S. stercoralis infective larvae on exposed skin and 70% ethanol may be used to disinfect contaminated surfaces.
Suggested Reading
Page, W., Judd, J. and Bradbury, R.S., 2018. The unique life cycle of Strongyloides stercoralis and implications for public health action. Tropical Medicine and Infectious Disease, 3 (2), p.53.
Watts, M.R., Robertson, G. and Bradbury, R.S., 2016. The laboratory diagnosis of Strongyloides stercoralis. Microbiology Australia, 37 (1), pp.4-9.
Toledo, R., Munoz-Antoli, C. and Esteban, J.G., 2015. Strongyloidiasis with emphasis on human infections and its different clinical forms. In Advances in Parasitology (Vol. 88, pp. 165-241). Academic Press.
Grove, D.I., 1996. Human strongyloidiasis. In Advances in Parasitology (Vol. 38, pp. 251-309). Academic Press.
Ashford, R.W., Barnish, G. and Viney, M.E., 1992. Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitology Today, 8(9), pp.314-318.
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.