Gnathostomiasis
[Gnathostoma binucleatum] [Gnathostoma doloresi] [Gnathostoma hispidum] [Gnathostoma nipponicum] [Gnathostoma spinigerum]
Causal Agents
Gnathostoma spp. are spirurid nematodes characterized by the presence of a prominent cephalic bulb and body spines, and are typically associated with carnivorous mammal definitive hosts. Humans are accidental hosts; the only forms found in humans are larvae or immature adults that never reach reproductive maturity. Most human infections are caused by G. spinigerum; other species confirmed to be zoonotic include G. hispidum, G. doloresi, G. binucleatum, and G. nipponicum. Two unconfirmed human cases of G. malaysiae infection have been reported from Myanmar.
Life Cycle
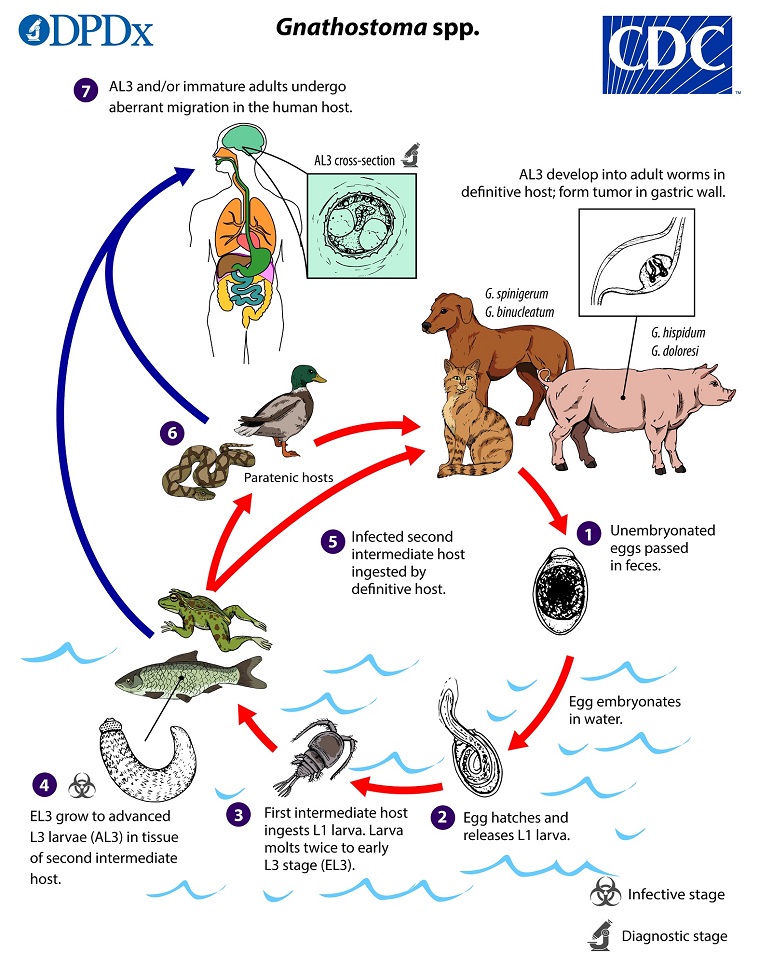
In definitive hosts, adult worms of most Gnathostoma spp reside in a tumor-like mass in the gastric wall; adult worms of some species are found in the esophagus or kidney. Adults mate and produce unembryonated eggs, which pass through a small opening in the tumor-like mass and ultimately into the feces  . Eggs become embryonated in water, and eggs release sheathed first-stage larvae (L1)
. Eggs become embryonated in water, and eggs release sheathed first-stage larvae (L1)  . Freshwater copepods, which serve as first intermediate hosts, ingest the free-swimming L1, and the larvae molt twice to become early third-stage larvae (EL3)
. Freshwater copepods, which serve as first intermediate hosts, ingest the free-swimming L1, and the larvae molt twice to become early third-stage larvae (EL3)  . Following ingestion of the copepod by a suitable second intermediate host, the EL3 migrate into the tissues of the host and develop further into advanced L3 larvae (AL3)
. Following ingestion of the copepod by a suitable second intermediate host, the EL3 migrate into the tissues of the host and develop further into advanced L3 larvae (AL3)  . When the second intermediate host is ingested by a definitive host, the AL3 develop into adult parasites in the gastric wall
. When the second intermediate host is ingested by a definitive host, the AL3 develop into adult parasites in the gastric wall  . Alternatively, the second intermediate host may be ingested by a paratenic host, in which the AL3 do not develop further but remain infective
. Alternatively, the second intermediate host may be ingested by a paratenic host, in which the AL3 do not develop further but remain infective  . Humans become infected by eating raw or undercooked meat of second intermediate or paratenic hosts containing AL3. In the human host, AL3 migrate in various tissues and may develop into immature adults but never achieve reproductive maturity; they may range in size from 2 mm to about 2 cm depending on the species and the extent of development
. Humans become infected by eating raw or undercooked meat of second intermediate or paratenic hosts containing AL3. In the human host, AL3 migrate in various tissues and may develop into immature adults but never achieve reproductive maturity; they may range in size from 2 mm to about 2 cm depending on the species and the extent of development  . Whether humans can become infected by drinking water that contains infected copepods is not clear.
. Whether humans can become infected by drinking water that contains infected copepods is not clear.
Hosts
Carnivorous and sometimes omnivorous mammals serve as definitive hosts for Gnathostoma spp., including canids and felids for G. spinigerum and G. binucleatum, swine for G. hispidum and G. doloresi, and weasels for G. nipponicum.
Several members of the freshwater copepod family Cyclopidae appear to be competent first intermediate hosts. Gnathostoma spp. larvae have been found in a broad range of second intermediate and paratenic hosts. Second intermediate hosts are typically aquatic animals that feed on copepods (e.g., fish, amphibians), and paratenic hosts are usually animals that prey on these second intermediate hosts (e.g., snakes, birds).
Geographic Distribution
Most human cases of gnathostomiasis are reported from Gnathostoma-endemic regions in which raw fish dishes (e.g., sushi, ceviche) are popular, particularly in Japan, Thailand, Vietnam, and Mexico. In the United States, cases of gnathostomiasis are most commonly reported in Southeast Asian immigrants.
Gnathostoma spinigerum, G. doloresi, and G. hispidum are endemic in East and Southeast Asia. In tropical Australia, G. spinigerum and G. hispidum have been reported sporadically in definitive hosts and several human cases of Gnathostoma infection that were diagnosed solely on the basis of serologic testing have been reported. G. doloresi is found in swine in parts of Central and Eastern Europe. G. nipponicum has been identified in Japan and China only. Occasional cases of gnathostomiasis associated with exposures in Africa (Zambia, Botswana, and Tanzania) have been reported; the causative species was identified for only one of the cases (as G. spinigerum for a case in a tourist who had visited Botswana).
Several Gnathostoma spp. occur in New World animal hosts. However, the only species known to be zoonotic in this range is G. binucleatum, a species that has considerable morphologic overlap with G. spinigerum, and has been implicated as the cause of many human cases of gnathostomiasis in Mexico and Ecuador. On the basis of serologic evidence, G. binucleatum may have caused additional cases of gnathostomiasis in other South American countries. On the basis of molecular data, the reported Latin American cases of gnathostomiasis historically attributed to G. spinigerum or G. spinigerum-like species were most likely caused by G. binucleatum.
Clinical Presentation
The clinical manifestations in humans are caused by migration of the advanced L3 larvae or immature adults, which can invade a variety of tissues. Eosinophilia is commonly observed during initial larval migration (e.g., in the skin and subcutaneous tissue) but is not always present in chronically infected persons. Cutaneous gnathostomiasis, the most common form of the disease, results from migration of the parasite in subcutaneous tissue, which can be associated with intermittent migratory swellings (often on the torso or upper limbs). These swellings can be erythematous, pruritic, and/or painful and may recur over months or years if the infection is not treated. In some patients, the parasite migrates near the surface of the skin and can be extracted or may spontaneously emerge.
Migration in deeper tissues (such as pulmonary, gastrointestinal, genitourinary, auricular, ocular tissue, or the central nervous system) is referred to as visceral gnathostomiasis or larva migrans profundus. Clinical manifestations are highly variable and depend in part on which tissues are affected. Neurognathostomiasis can be associated with potentially fatal eosinophilic meningitis and myeloencephalitis, and ocular gnathostomiasis may cause vision loss.
Scanning electron micrographs of a Gnathostoma spinigerum female worm, showing the cuticular armature of the body surface. The cuticular armature is important for identification of Gnathostoma spp.





Cross sections of immature Gnathostoma spp. stained with hematoxylin and eosin (H&E).
Diagnostic characteristics of immature Gnathostoma worms found in humans include the presence of large, cavernous lateral chords; multinucleated intestinal cells (some species); pigmented granular material in the intestinal cells; and spines on the cuticle (although some species do not have spines on the entire length of the body). In histologic sections, some Gnathostoma spp. can be differentiated from each other on the basis of the morphologic features of their intestinal epithelial cells.









Morphologic Diagnosis
Morphologic diagnosis is achieved by identifying Gnathostoma larval forms or immature adults in a biopsy specimen or after extraction from a cutaneous lesion. In histologic sections, Gnathostoma spp. often can be differentiated from each other on the basis of the morphologic features of their intestinal epithelial cells.
Antibody Detection
Serologic tests for Gnathostoma infection are not available at CDC or elsewhere in the United States. Please contact DPDx for contact information for laboratories in Thailand and Japan that perform serologic testing.
Laboratory Safety
Standard laboratory precautions apply for the processing and examination of histologic sections. No stages of Gnathostoma spp. examined in diagnostic laboratories represent an infectious risk.
Suggested Reading
Dekumyoy, P., Yoonuan, T., Waikagul, J., 2013. Gnathostoma. Molecular detection of parasitic pathogens. Boca Raton: CRC Press, pp.563-570.
Herman, J.S. and Chiodini, P.L., 2009. Gnathostomiasis, another emerging imported disease. Clinical Microbiology Reviews, 22 (3), pp.484-492.
Nawa, Y., Yoshikawa, M., Sawanyawisuth, K., Chotmongkol, V., Figueiras, S.F., Benavides, M. and Camacho, S.P.D., 2017. Ocular gnathostomiasis—update of earlier survey. The American Journal of Tropical Medicine and Hygiene, 97 (4), pp.1232-1234.
Rusnak, J.M. and Lucey, D.R., 1993. Clinical gnathostomiasis: case report and review of the English-language literature. Clinical Infectious Diseases, 16(1), pp.33-50.
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.