
Hookworm (Intestinal)
[Ancylostoma duodenale][Ancylostoma ceylanicum][Necator americanus]
Causal Agents
Intestinal hookworm disease in humans is caused by Ancylostoma duodenale, A. ceylanicum, and Necator americanus. Classically, A. duodenale and N. americanus were considered the two primary intestinal hookworm species worldwide, but newer studies show that a parasite infecting animals, A. ceylanicum, is also an important emerging parasite infecting humans in some regions. Occasionally larvae of A. caninum, normally a parasite of canids, may partially develop in the human intestine and cause eosinophilic enteritis, but this species does not appear to reach reproductive maturity in humans.
Another group of hookworms infecting animals can penetrate the human skin causing cutaneous larva migrans (A. braziliense, A. caninum, Uncinaria stenocephala). Other than A. caninum noted above, these parasites do not develop further after their larvae penetrate human skin. See extraintestinal hookworms for more information.
Life Cycle
Eggs are passed in the stool  , and under favorable conditions (moisture, warmth, shade), larvae hatch in 1 to 2 days and become free-living in contaminated soil. These released rhabditiform larvae grow in the feces and/or the soil
, and under favorable conditions (moisture, warmth, shade), larvae hatch in 1 to 2 days and become free-living in contaminated soil. These released rhabditiform larvae grow in the feces and/or the soil  , and after 5 to 10 days (and two molts) they become filariform (third-stage) larvae that are infective
, and after 5 to 10 days (and two molts) they become filariform (third-stage) larvae that are infective  . These infective larvae can survive 3 to 4 weeks in favorable environmental conditions. On contact with the human host, typically bare feet, the larvae penetrate the skin and are carried through the blood vessels to the heart and then to the lungs. They penetrate into the pulmonary alveoli, ascend the bronchial tree to the pharynx, and are swallowed
. These infective larvae can survive 3 to 4 weeks in favorable environmental conditions. On contact with the human host, typically bare feet, the larvae penetrate the skin and are carried through the blood vessels to the heart and then to the lungs. They penetrate into the pulmonary alveoli, ascend the bronchial tree to the pharynx, and are swallowed  . The larvae reach the jejunum of the small intestine, where they reside and mature into adults. Adult worms live in the lumen of the small intestine, typically the distal jejunum, where they attach to the intestinal wall with resultant blood loss by the host
. The larvae reach the jejunum of the small intestine, where they reside and mature into adults. Adult worms live in the lumen of the small intestine, typically the distal jejunum, where they attach to the intestinal wall with resultant blood loss by the host  . Most adult worms are eliminated in 1 to 2 years, but the longevity may reach several years.
. Most adult worms are eliminated in 1 to 2 years, but the longevity may reach several years.
Some A. duodenale larvae, following penetration of the host skin, can become dormant (hypobiosis in the intestine or muscle). These larvae are capable of re-activating and establishing patent, intestinal infections. In addition, infection by A. duodenale may probably also occur by the oral and the transmammary route. A. ceylanicum and A. caninum infections may also be acquired by oral ingestion. A. caninum-associated eosinophilic enteritis is believed to result following oral ingestion of larvae, not percutaneous infection. N. americanus does not appear to be infective via the oral or transmammary route.
Hosts
Humans are the principal host for both A. duodenale and N. americanus. A. ceylanicum may be zoonotic, as two haplotypes have been identified, one found only in humans thus far and the other found in humans, dogs, and cats. A. caninum is the common dog hookworm.
Geographic Distribution
Hookworm species have a worldwide distribution, mostly in areas with moist, warm climates where larvae can survive in the environment. Both Necator americanus and Ancylostoma duodenale are found in Africa, Asia, Australia and the Americas. Only N. americanus is found in south India and predominates in the Americas, while only A. duodenale is found in the Middle East, North Africa, and northern India.
A. ceylanicum is highly endemic throughout much of Southeast Asia and the Pacific Islands, and also has been reported from Australia, Japan, South Africa, Madagascar, Suriname, Guyana, and the UAE; it appears to be absent from Europe and North America. However, the full extent of its geographic occurrence is not completely characterized.
Clinical Presentation
Intestinal hookworm infections are commonly asymptomatic. Attachment of the hookworms to the intestinal wall may stimulate abdominal pain, nausea, and anorexia. Iron deficiency anemia caused by blood loss at the site of intestinal attachment of adult worms may occur especially in heavy infections. Occult blood in the stool may also be seen in heavy infections. In severe cases, protein malnutrition from chronic plasma protein loss has been reported.
Other clinical manifestations of hookworm infection include an urticarial dermal reaction (“ground itch”) associated with filariform (L3) larvae penetration, and respiratory involvement including eosinophilic pneumonia may be observed may occur during larval pulmonary migration A second urticarial rash may subsequently develop during pulmonary migration. Patients have reported vague gastrointestinal disturbances and eosinophilia (sometimes referred to as Wakana syndrome) following peroral infection.
Hookworm eggs.

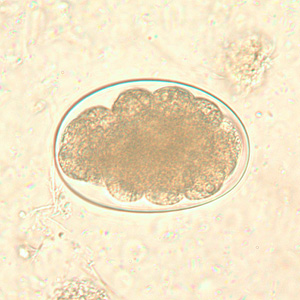




Hookworm rhabditiform larvae.


Hookworm filariform larvae.
Infective, third-stage (L3), filariform larvae are 500—700 µm long. They have a pointed tail and are ensheathed, with about a 1:2 ratio in length of esophagus to intestine. Some subtle morphological differences exist between A. duodenale and N. americanus at this stage. These L3 are found in the environment and infect the human host by penetration of the skin.




Adult hookworms.
Adult hookworms reside in the small intestine of their hosts. A. duodenale males measure approximately 8—12 mm long, and females measure approximately 10—15 mm long. N. americanus males are 5—9 mm long, females 9—11 mm. Males are bursate, with two spicules that are fused at the distal end in Necator spp. but not in Ancylostoma spp. Adults of both sexes have a buccal capsule containing sharp teeth (Ancylostoma) or cutting plates (Necator).


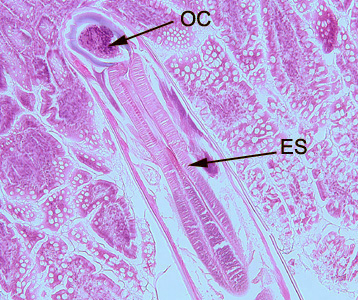
Hookworms in tissue, stained with hematoxylin and eosin (H&E).


Laboratory Diagnosis
Microscopic identification of eggs in the stool is the most common method for diagnosing hookworm infection. The recommended procedure is as follows:
- Collect a stool specimen.
- Fix the specimen in formalin.
- Concentrate using the formalin–ethyl acetate sedimentation technique.
- Examine a wet mount of the sediment.
Where concentration procedures are not available, a direct wet mount examination of the specimen is adequate for detecting moderate to heavy infections. For quantitative assessments of infection, various methods such as the Kato-Katz, FLOTAC and Mini-FLOTAC may be used.
Laboratory Safety
Standard protocols apply for the handling of stool specimens. Appropriate PPE (gloves, gown) should always be worn to minimize the risk of transdermal penetration when working with stool specimens or larval cultures. In vitro exposure to 70% ethanol has been shown to kill 95.6% of 45 infective N. americanus larvae within five minutes and to kill all such larvae within 10 minutes, making ethanol a suitable surface disinfectant. Lugol’s iodine (1% povidine iodine; 10,000 ppm) may be used to kill hookworm larvae on exposed skin.
Suggested Reading
Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG, 2018. Soil-transmitted helminth infections. Lancet, 391, pp. 252–65.
Traub, R.J., 2013. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. International Journal for Parasitology, 43(12-13), pp.1009–1015.
Hotez PJ. 2006. Chapter 116—Hookworm infections. In: Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases. New York: Saunders Elsevier; 2011, pp. 799–804.
Brooker, S., Bethony, J. and Hotez, P.J., 2004. Human hookworm infection in the 21st century. Advances in Parasitology, 58, p.197.
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.
