The AMR Challenge

U.S. Secretary of Health and Human Services Alex Azar speaking at the 2018 AMR Challenge.
Held September 2018 to September 2019
The Antimicrobial Resistance (AMR) Challenge was a yearlong effort by the U.S. government to accelerate the fight against AMR. The Challenge resulted in more than 350 organizations across the globe committing to slow AMR.
The AMR Challenge encouraged a One Health approach, recognizing that the health of people is connected to the health of animals and the environment. CDC, on behalf of the U.S. government, encouraged commitments that focused on at least one of the five commitment areas:
chart icon Tracking and data: Share data and improve data collection
lab icon Infection prevention and control: Reduce the spread of resistant germs
medical icon Antibiotic use: Improve antibiotic use, including ensuring people can access these medicines when needed
allergy icon Environment and sanitation: Decrease antibiotics and resistance in the environment, including improving sanitation
medical icon Vaccines, therapeutics, and diagnostics: Invest in development and improved access
The Challenge kicked off and concluded at United Nations (UN) General Assembly side events in 2018 and 2019. The events were attended by representatives from governments, private industry, and organizations committed to keeping AMR a priority.
The events were filmed live and are available on YouTube:
Global Participation in the AMR Challenge
Commitments were made in 32 countries. The image below shows the number of commitments by country, updated December 2019.
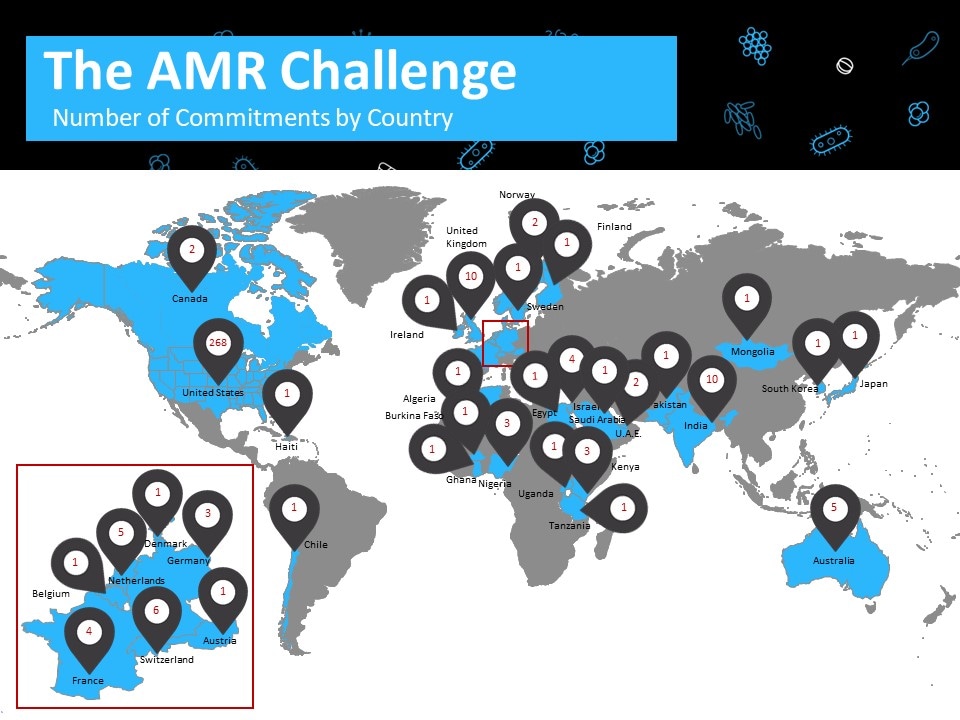
| Country | Number of Commitments |
|---|---|
| Algeria | 1 |
| Australia | 5 |
| Austria | 1 |
| Belgium | 1 |
| Burkina Faso | 1 |
| Canada | 2 |
| Chile | 1 |
| Denmark | 1 |
| Finland | 1 |
| France | 4 |
| Germany | 3 |
| Ghana | 1 |
| Haiti | 1 |
| India | 10 |
| Ireland | 1 |
| Israel | 4 |
| Japan | 1 |
| Kenya | 3 |
| Mongolia | 1 |
| Netherlands | 5 |
| Nigeria | 3 |
| Norway | 2 |
| Pakistan | 1 |
| Saudi Arabia | 1 |
| South Korea | 1 |
| Sweden | 1 |
| Switzerland | 6 |
| Tanzania | 1 |
| Uganda | 1 |
| United Arab Emirates | 2 |
| United Kingdom | 10 |
| United States | 268 |
Commitments Made to the AMR Challenge
Nearly 350 commitments were made during the Challenge to accelerate the fight against AMR. Although CDC is no longer accepting commitments, please email if interested in combating AMR together: ARX@cdc.gov. The list below was updated October 2019.
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
98point6 (Washington, U.S.)
98point6—an on-demand, text-based, virtual primary care application—commits to continue establishing antibiotic stewardship as a key performance indicator within its practice. The 98point6 Clinical Quality Assurance team tracks adherence to antibiotic guidelines and reports this data across the company and to partners. By year-end, the company plans to build and implement consistent ways of communicating the importance of proper antibiotic usage to providers and patients, as well as expand practice standards and clinic training in this area. When patients request antibiotics, 98point6 clinicians use standardized antibiotic stewardship language. After visits, patients receive robust information about antibiotics within the application.
5D Health Protection Group Ltd (United Kingdom)
The 5D Health Protection Group Ltd commits to developing new antimicrobial and antibiofilm agents by 2022. These new antimicrobial and antibiofilm technologies will treat wound and medical device related infections, and will provide an alternative treatment to antibiotic classes in which bacterial resistance is high. By providing alternative treatments to existing antibiotics, it is anticipated that the global use of antibiotics could be decreased significantly.
Abt Associates Inc. (Massachusetts, U.S.)
Abt Associates—a global company that conducts research, consulting, and technical services in health, environmental and social policy, technology, and international development—commits to pursuing opportunities within new and existing global projects to conduct antibiotic resistance mapping, landscaping, and trend analysis. In particular, Abt Associates aims to help better understand the status and threat of antibiotic resistance among private health facilities and pharmacies worldwide, among formal and informal providers, and to collect data on contextual factors that contribute to antibiotic resistance in particular geographic regions. Abt Associates plans to share resistance data, news, and information from these projects via multiple external communication channels and existing project reporting relationships to CDC and USAID.
Accelerate Diagnostics, Inc. (Arizona, U.S.)
Accelerate Diagnostics, Inc. commits to investing nearly $100 million to develop rapid diagnostics to detect resistant bloodstream, respiratory, and other serious bacterial infections, enabling clinicians to identify organisms and tailor antibiotic therapy days earlier than is currently possible. These diagnostic solutions, the first of which is currently in use in hospitals around the world for bloodstream infections, will help clinicians tailor therapy for 5 million patients within the next 5 years.
AdvaMedDx (Washington, D.C., U.S.)
AdvaMedDx is committed to improving patient care and public health through increased access to appropriately used diagnostic tests for early detection of resistant infections, informed prescribing of antibiotics, and surveillance in the fight against antimicrobial resistance (AMR). Representing the world’s largest diagnostics manufacturers, AdvaMedDx will advocate for policies that incentivize and support robust stewardship programs that are informed by diagnostic data; engage with provider and professional organizations to disseminate educational materials that encourage the appropriate use of diagnostics in antibiotic stewardship programs; and continue to engage with policy makers in the U.S. and globally to raise awareness of AMR and to educate them on the benefits of policy changes that incentivize robust antibiotic stewardship programs.
Adventist Development and Relief Agency
Adventist Development and Relief Agency (ADRA), a humanitarian organization providing long-term development programs and emergency response internationally, commits to working with its 130 national offices, partners, stakeholders, and donors to raise awareness for equal access to safe water, adequate sanitation, and a hygienic environment in all healthcare facilities. Additionally, ADRA commits to providing healthcare services that respect the dignity of all users and staff in order to improve health outcomes.
Aequor (San Diego, U.S.)
Aequor, Inc.—a manufacturer of treatments that prevent the ability of bacteria to form biofilm that causes infections—commits to increasing awareness of the importance of antimicrobial resistance, biofilm, infection prevention and control, and the One Health Initiative through more than 19 in-person events throughout 2019 and social media. Aequor also commits to researching and developing new antimicrobial therapeutics targeting the CDC’s and World Health Organization’s critical and high priority pathogens.
Aetna (Connecticut, U.S.)
As one of the largest health insurers in the U.S.—with 1.2 million health professionals, more than 5,700 hospitals, and 22.2 million members—Aetna is committed to the dual goals of reducing inappropriate antibiotic prescribing and promoting the appropriate use of vaccinations among its members. We do that by partnering with state health departments to offer feedback to providers about their antibiotic prescribing performance, integrating stewardship metrics in our value-based contracts, and promoting vaccinations.
Africa Christian Health Associations Platform (Maryland, U.S.)
Africa Christian Health Associations Platform (ACHAP), 43-member Christian Health Associations/Networks with more than 5,000 healthcare facilities in 32 countries in sub-Saharan Africa, commits to promoting safe drinking water, sanitation, and hygiene (WASH) alliance building in order to deliver better care for patients. ACHAP commits to contributing to policy dialogue and implementation; sharing expertise, experiences and success stories; strengthening health systems; instituting WASH mentorship and coaching programs; and promoting social accountability mechanisms to ensure community participation and ownership for WASH in healthcare settings. ACHAP commits to undertaking research, analysis, advocacy, and communications; building culture and consciousness to ensure sustainability and resilience in health services; and promoting WASH as a tool towards attaining Universal Health Coverage.
Alabama Department of Public Health (Alabama, U.S.)
The Alabama Department of Public Health’s Healthcare-Associated Infections Program and Antimicrobial Stewardship Program commit to collaborating with state partners such as the Alabama Hospital Association (AlaHA), the Alabama Quality Assurance Foundation (AQAF), and the Alabama Nursing Home Association (ANHA), as well as Alabama’s healthcare facilities, to support infection prevention efforts and improve antibiotic prescribing and use throughout the state. This will be executed by providing trainings, webinars, and newly developed educational tools for healthcare workers and patients on appropriate antibiotic use and antibiotic resistance.
Alaska Department of Health and Social Services (Alaska, U.S.)
The Alaska Department of Health and Social Services commits to addressing the threat of antimicrobial resistance by increasing laboratory capacity for early detection, rapidly initiating infection containment response measures, and preventing the spread of infection to Alaska residents. Working with the Alaska Antimicrobial Stewardship Collaborative, the department provides education, resources, guidelines, and regional antibiograms to assist its clinicians in choosing the best antibiotic treatment for patients.
ALK (Denmark)
Through educational programs and onsite support, ALK, a global pharmaceutical company, commits to educating healthcare providers in inpatient and outpatient facilities across the U.S. on implementation of penicillin allergy assessment services. According to CDC, 10% of the U.S. population reports a penicillin allergy but less than 1% are truly allergic. Implementation of these services could reduce the unnecessary use of broad spectrum and sub-optimal antibiotics and contribute to improved patient safety. ALK supports the National Penicillin Allergy Day campaign and strives to see increased participation from healthcare providers and practices in 2019.
American Academy of Emergency Medicine (Wisconsin, U.S.)
The American Academy of Emergency Medicine (AAEM), a professional society with over 8,000 emergency physician members, commits to developing and promoting the first antibiotic stewardship pledge for emergency care providers. This pledge incorporates stewardship principles relevant to the emergency department setting. In addition to featuring a general commitment to improve antibiotic prescribing, the pledge includes condition-specific statements aimed at optimizing care for infections frequently treated in the emergency department such as sepsis, respiratory tract infections, and urinary tract infections. AAEM will also regularly incorporate infectious disease educational topics into continuing education offerings such as its annual Scientific Assembly.
American Academy of Family Physicians (Kansas, U.S.)
The American Academy of Family Physicians (AAFP), one of the nation’s largest medical organizations with 134,600 members, commits to educating its membership on the importance of antibiotic stewardship. AAFP will continue to highlight the importance of antibiotic stewardship through continuing medical education and non-medical education opportunities at conferences, through webinars, in journal articles, and in-person courses. AAFP will also continue to promote antibiotic stewardship through various ways to increase awareness.
American Academy of Orthopaedic Surgeons (Illinois, U.S.)
The American Academy of Orthopaedic Surgeons (AAOS) will increase awareness among its 38,000 members in more than 100 countries of evidence-based strategies to prevent and treat surgical site infections and periprosthetic joint infections. AAOS will also increase awareness of when antibiotics should and should not be used for patients with hip and knee implants who are undergoing dental procedures.
American Academy of Pediatrics (Illinois, U.S.)
The American Academy of Pediatrics (AAP)—representing 67,000 pediatricians in the U.S.—commits to promoting antibiotic stewardship through policy and quality improvement practices. AAP will use antibiotic use quality measures to improve use in inpatient and outpatient settings and share antibiotic use information with physicians and patients, including a pediatrician toolkit for all settings. AAP will promote vaccines to prevent bacterial infections that often lead to antibiotic use through policy, education, and advocacy targeting pediatricians and families. AAP will engage AAP Chapters in the Chapter Quality Network to implement outpatient quality improvements and collaborate to create antibiotic resistance strategies.
American Animal Hospital Association (Colorado, U.S.)
The American Animal Hospital Association (AAHA), the accrediting body for companion animal practices in the U.S. and Canada, commits to educating the profession on antibiotic use through the just-released AAHA Infection Control, Prevention and Biosecurity (ICPB) Guidelines, updates to the AAHA Standards of Accreditation, and review and revision of the Judicious Use of Antimicrobials position statement. Revisions will reflect findings by the Task Force on Antimicrobial Stewardship in Companion Animal Practice and the American Veterinary Medical Association Committee on Antimicrobials. AAHA will also have educational tracks at numerous veterinary conferences in 2019 to educate and inform veterinary colleagues about the new ICPB guidelines to promote the highest standard of care for animals. The AAHA Standards of Accreditation include many standards related to infection prevention and control, sanitation, and stewardship.
American Association of Avian Pathologists (Florida, U.S.)
The American Association of Avian Pathologists (AAAP) commits to maintaining poultry health and welfare by implementing strategies to prevent, control, and treat common diseases by using an evidence-based approach in antimicrobial decisions, then using antimicrobials judiciously and with continual evaluation of the outcomes of therapy while protecting poultry health and ensuring safe, affordable food to the consumer. Also as part of its mission is to promote scientific knowledge to enhance the health, well-being, and productivity of poultry to provide safe and abundant food for the world, AAAP is committed to collecting and tracking on-farm antimicrobial use data by AAAP veterinarians who are assisting with a project to quantify on-farm antimicrobial use within the U.S. poultry industry.
American Association of Nurse Practitioners (Texas, U.S.)
The American Association of Nurse Practitioners (AANP), representing 270,000 nurse practitioners across the United States, commits to raising awareness of infection prevention and appropriate antibiotic use among nurse practitioners and patients. AANP will provide its members with information about antibiotic stewardship in its two peer-reviewed journals (The Journal of the American Association of Nurse Practitioners and The Journal for Nurse Practitioners), newsletters, website, social media, and conferences throughout 2020. Additionally, patients will receive messaging about improving antibiotic use through AANP’s consumer-facing website.
American Association of Swine Veterinarians (Iowa, U.S.)
The American Association of Swine Veterinarians (AASV), a professional association with more than 1,500 members in more than 40 countries, commits to promoting antibiotic stewardship among swine veterinarians by providing resources with information on appropriate antibiotic use. AASV will advocate for science-based approaches to veterinary, industry, and public health issues, including antimicrobial resistance. AASV also commits to promoting veterinary oversight, the use of data collection, and disease prevention.
American Cancer Society (Georgia, U.S.)
The American Cancer Society commits to increasing patient awareness of the importance of infection prevention and control, as well as antibiotic resistance, through website and blog communications materials.
American College of Physicians (Pennsylvania, U.S.)
With 154,000 members of internal medicine physicians, medical students, residents, and fellows, the American College of Physicians (ACP) will continue incorporating evidence about antibiotic stewardship into clinical policies relevant to antibiotic treatment and prophylaxis, and publish an opinion piece in a peer-reviewed journal to spread awareness of the urgency of the issue.
American Dental Association (Illinois, U.S.)
The American Dental Association (ADA) commits to creating and disseminating guidance to help clinicians appropriately prescribe antibiotics for dental pain and swelling. The ADA also commits to publishing a survey of current antibiotic prescribing practices among dentists to demonstrate the need for such guidance.
American Health Care Association (Washington, DC, U.S.)
American Health Care Association (AHCA) commits to sharing infection prevention and control and antibiotic stewardship related survey citation data as well as long-term and post-acute care setting specific operational perspectives with the CDC. AHCA strongly supports antibiotic stewardship as a national priority. The AHCA Quality Initiative calls on members to pursue quality improvement in several key areas including reducing unnecessary hospitalizations, which can involve inappropriate antibiotic use and improving identification and management of infections. Identifying successful strategies for implementing antibiotic stewardship will enable CDC in collaboration with AHCA members to promote activities to improve how antibiotics are used in the care of frail and older adults in post-acute and long-term care settings. AHCA and members have been active participants providing valuable insight at a number of stakeholder meetings on antibiotic stewardship at both state and national levels.
American Hospital Association (Illinois, U.S.)
The American Hospital Association (AHA) will work with its members to prevent infections and reduce the spread of germs, and improve the use of antibiotics wherever they are used. Specifically, AHA will support hospital members to improve antibiotic use, solicit commitments to implement targeted assessments for infection prevention (TAP), support containment of emerging resistance with state and local health departments, and provide patient education on antimicrobial resistance. AHA represents nearly 5,000 member hospitals, health systems, and other health care organizations; clinical partners–including more than 270,000 affiliated physicians, 2 million nurses and other caregivers; and 43,000 health care leaders belonging to their professional membership groups.
American Public Health Association (APHA) (Washington, D.C., U.S.)
The American Public Health Association (APHA) commits to raising awareness through communications channels, The Nation’s Health, in-person events and social media among its 25,000 members of how antibiotic resistance places public health at risk, and what steps public health professionals can take to be part of the solution. APHA advocates for a One Health approach, focusing on human and animal health, and will continue to advocate for increased public health funding on antibiotic resistance.
American Society of Consultant Pharmacists (Virginia, U.S.)
The American Society of Consultant Pharmacists (ASCP) is an international association representing more than 8,000 pharmacy professionals and students who serve the unique medication needs of older adults. ASCP commits to promoting antibiotic stewardship across the spectrum of older adult care through practice resource development, industry partnerships, educational materials, and proactive market research and support.
American Society for Microbiology (Washington, D.C., U.S.)
With its 30,000 members in 161 countries, the American Society for Microbiology (ASM) is committed to working with global stakeholders to develop and execute a roadmap for surveillance and monitoring of antibiotic use and antibiotic resistance. ASM currently works across countries and sectors to establish, bring to scale, and sustainably maintain such systems by focusing on strengthened laboratory capacity and global health security programs in low resourced settings.
American Society of Health-System Pharmacists (Maryland, U.S.)
The American Society of Health-System Pharmacists (ASHP) commits to education, practice standards, certification, and consultation related to antibiotic use for its over 45,000 members, including pharmacists, student pharmacists, and pharmacy technicians. ASHP also continues to offer a broad range of education, training, and certification resources at ASHP-hosted conferences and online. In addition, ASHP will create an antibiotic stewardship certificate on developing and optimizing stewardship programs that will be released in 2019. ASHP also offers a consulting service to assist hospitals and health systems with the development or enhancement of stewardship programs.
American Telemedicine Association (Virginia, U.S.)
American Telemedicine Association (ATA), working to advance health through telecommunications technology (called telehealth), commits to engaging its 400-member United States network to conduct trend analysis of antibiotic use and identify best practices in antibiotic drug prescribing. Data collected will be used to facilitate member trainings, webinars, and other educational offerings to improve compliance with antibiotic stewardship. ATA is working to ensure that safe, affordable, and appropriate care is available when and where it is need for their patients.
American Urological Association (Maryland, U.S.)
The American Urological Association (AUA) commits to partnering with CDC to generate and share data to inform antibiotic use strategies to reduce resistant infections following prostate biopsies. This partnership will allow for large dataset analyses that will provide evidence to potentially inform more granular recommendations on the best approaches to prevent such infections. Such approaches may include tailoring surgical techniques and/or antibiotic prophylaxis to patient-level factors and local antibiotic resistance patterns. The data may also provide evidence that can aid clinical management of other infections as well (for example, urinary tract infections). This effort is expected to take 12-18 months.
American Veterinary Medical Association (Illinois, U.S.)
The American Veterinary Medical Association (AVMA) advocates for stronger veterinary oversight and responsible use of antibiotics to help protect the health of animals and people, animal welfare, and the food supply. With more than 91,000 members, AVMA’s efforts will support common understanding of the critical role of effective antibiotic stewardship in combating the development of antibiotic resistance through: ongoing collaboration with the Food and Drug Administration to achieve enhanced veterinary oversight of antibiotic use; education of the veterinary profession on antibiotic stewardship; assistance with enhanced surveillance of antibiotic use in animals; support for research; and more active engagement with international stakeholders.
AMR Centre (United Kingdom)
The AMR Centre (AMRC), the research and development centre for new antibiotics and diagnostics in the United Kingdom, commits to researching and developing new and novel antibiotic therapeutics, targeting CDC’s and the World Health Organization’s critical priority pathogens. AMRC offers research and development from pre-clinical leads to clinical proof of concept to bring new technologies to patients suffering from life-threatening drug-resistant infections. AMRC is currently progressing three new antibiotic projects including a T3SS inhibitor (pneumonia), metallo-beta-lactamase inhibitor (gram-pathogens), and an antimicrobial peptide (gram-pathogens), with another seven projects under review.
AMR Industry Alliance (Switzerland)
The AMR Industry Alliance brings together over 100 biotech, diagnostic, generic, and research-based pharmaceutical companies around the shared goal of curbing antimicrobial resistance (AMR) in the world. Alliance companies are committed to contribute to and measure their efforts in fighting AMR across four key areas: research, appropriate use, access, and manufacturing and the environment. Earlier in 2018, AMR Alliance generic and research-based pharmaceutical companies agreed on a framework that promotes responsible antibiotic manufacturing. These companies took a further step by publishing the first list of discharge targets to guide environmental risk assessments for the manufacture of antibiotics. This publication is an important step in the journey as companies work toward achieving these target values. The Alliance companies will continue to take action and share their experiences to reduce environmental impact from the production of antibiotics.
AMR Insights (Netherlands)
AMR Insights commits to increasing the public professional awareness of the threat of antimicrobial resistance (AMR) by sharing knowledge and connecting experts through their network of up to 50,000 subscribers of their bimonthly newsletter. AMR Insights commits to developing and implementing a globally accessible database with emerging technologies in AMR. They will also convene an annual meeting titled “Emerging Technologies in AMR” with participants from around the world.
Animal Health Institute (Washington, D.C., U.S.)
The Animal Health Institute (AHI), an organization representing twelve companies in veterinary health, commits to working with the U.S. Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA) to improve the responsible use of antibiotics in food-producing and companion animals and to create science-based measurements that help veterinarians prescribe antibiotics responsibly. AHI will support work to develop innovative tools—including disease prevention and biosecurity practices, vaccines, new medical therapies, and alternatives to traditional antibiotics—to help veterinarians meet animal disease challenges. AHI will serve as a resource to policy makers and stakeholders providing technical information on animal health products and champion efforts to support science and evidence-based solutions.
Anthem (Indiana, U.S.)
Improving patient health by reducing inappropriate use of antibiotics is important to Anthem. By including antimicrobial resistance metrics in their value-based arrangements for both hospitals and physicians, Anthem commits to working with and financially rewarding providers for high and improved performance. This commitment includes working with care delivery transformation teams who interact directly with physicians to improve member education.
Antibiotic Resistance Action Center (Washington, D.C., U.S.)
The Antibiotic Resistance Action Center (ARAC) at the Milken Institute School of Public Health, George Washington University, commits to advancing knowledge of how antibiotic use in food animals impacts human health and to improving antibiotic prescribing in the urgent care sector. ARAC is studying the impact of new California legislation, which limits the use of antibiotics in livestock, by testing purchased meat and human biological samples from the region for antibiotic-resistant germs that can cause infections. ARAC is also launching an awareness campaign in 24 U.S. urgent care clinics during flu season to reduce inappropriate antibiotic use, assessing the impact of patient education materials through a cluster randomized study, and launching two summits to generate a strategy for urgent care antibiotic use standards.
Antimicrobial Stewardship Collaborative of South Carolina (South Carolina, U.S.)
The Antimicrobial Stewardship Collaborative of South Carolina (ASC-SC), the coordinator of antimicrobial stewardship and infection prevention efforts across the state, commits to collaborating with its partner facilities to advance local antimicrobial stewardship programs by implementing CDC’s Core Elements of Antimicrobial Stewardship. ASC-SC will encourage reporting of antimicrobial use and resistance with a goal of 50 percent increase in the number of acute care hospitals in South Carolina reporting either antimicrobial use or resistance to CDC’s National Health and Safety Network modules by 2020. ASC-SC also commits to improving antimicrobial use in the community by collecting and tracking ambulatory antimicrobial use utilization in South Carolina. ASC-SC will also partner with healthcare providers and professional organizations in ambulatory settings to disseminate educational materials that encourage appropriate use of antimicrobials for community-acquired infections with a goal of 10 percent overall reduction in ambulatory antimicrobial use by 2022.
Applied Silver, Inc. (California, U.S.)
Applied Silver, a health technology company, commits to sharing findings on the importance of hygiene in soft surfaces (e.g. fabrics and linens) in the healthcare environment at the American Society of Microbiology annual conference in June 2019. By spreading awareness of cleanliness in soft surfaces in hospitals, Applied Silver will reduce the spread of resistant pathogens. Applied Silver will advance efforts—including continuing education courses, webinars, workshops, and distribution of educational materials—to educate hospital staff, patients, regulatory agencies, and the community on the importance of incorporating soft surfaces interventions in standard infection control protocols in healthcare.
AquaTabs, Medentech, and Impact Water (Ireland)
AquaTabs and Impact Water commit to expanding their reach to healthcare facilities and hospitals within the second half of 2019. Their solutions include water and environmental control, and safe drinking water. World Vision and Impact Water have selected a representative sample of healthcare facilities to implement its solutions from August to October with the objective to assess the most appropriate service and solution mix, and will report on its measures.
Argonne National Laboratory (Illinois, U.S.)
Argonne National Laboratory commits to conducting research for improving detection of antimicrobial resistance mechanisms and prediction of antimicrobial susceptibility. First, by developing a panel that can detect hundreds of antimicrobial resistance genes at once (September 2019). Second, using machine learning (a subset of artificial intelligence) to identify genetic markers for sequence-based prediction of antimicrobial susceptibility. This includes ongoing efforts to collect and integrate phenotypic susceptibility data for more than 38,000 publically available genomes by September 2019. As part of this, Argonne is collaborating with CDC on better predicting resistance in Clostridioides difficile.
Arizona Department of Health Services (Arizona, U.S.)
The Arizona Department of Health Services commits to partnering with an antimicrobial stewardship expert from the University of Arizona to improve antibiotic use in 11 long-term care facilities. Data shared by healthcare facilities will be analyzed to understand current antibiotic susceptibility patterns (test results that show an antibiotic will be effective against a germ) and prescribing practices in Arizona. Findings and facility rankings will be shared among participating facilities. The department aims to encourage the responsible antibiotic use and educate on appropriate prescribing to improve patient outcomes, reduce antibiotic resistance, and decrease infections caused by resistant germs.
Arkansas Department of Health (Arkansas, U.S.)
The Arkansas Department of Health (ADH) will conduct more than 50 visits at Arkansas hospitals and nursing homes, evaluating practices and providing guidance to healthcare workers to bolster infection prevention and antibiotic stewardship programs during 2019. To prevent the spread of pathogens, ADH also commits to using results from a recent prevention effort to improve communication related to multidrug-resistant organisms and Clostridioides difficile when patients are transferred between healthcare facilities. Lastly, ADH commits to supporting educational opportunities to improve awareness of antibiotic resistance, including co-hosting a statewide symposium on antibiotic resistance and stewardship in November 2019.
Arkstone Medical Solutions (Florida, U.S.)
Arkstone Medical Solutions—a company providing the healthcare community with tools to reduce antimicrobial resistance—commits to monitoring antibiotic resistance, improving the inappropriate use of antibiotics, and reducing antibiotic-resistant infection rates. Arkstone Medical will share antibiotic resistance data gathered through their antimicrobial stewardship program with the public through monthly newsletters and weekly posts on social media.
Armenta Ltd. (Israel)
Armenta Ltd., an agricultural technology company, commits to developing novel, non-invasive, non-antibiotic therapies for dairy cattle diseases to reduce the use of antibiotics. Armenta aims to improve dairy herd health management with a focus on preventing losses resulting from mastitis, a common bacterial infection that causes inflammation in cattle milk ducts typically treated with antibiotics. Armenta developed a new therapy to treat mastitis with technology used in human healthcare. Armenta will continue to develop alternative therapies to help reduce the use of antibiotics, control infection, improve tissue function, and prevent the spread of antibiotic resistance.
Ascension (Missouri, U.S.)
As the nation’s largest nonprofit health system, Ascension is committed to working on improving antimicrobial use and the cleanliness of the healthcare environment to combat the threat of antimicrobial resistance (AMR) in its healthcare facilities. Ascension will: utilize CDC’s Targeted Assessment for Prevention (TAP) reports as a tool to improve infection prevention and antibiotic use; ensure cleaning and disinfection of the healthcare environment to decrease environmental exposure to antibiotics and resistance; reduce the risk of transmission of resistant germs by implementing appropriate isolation and hand hygiene procedures; utilize electronic health records to support the proper choice of antibiotics; and work with a coalition of health systems to increase the availability of poultry and meat raised without the routine use of antibiotics. Ascension set a goal for fiscal year 2019 is to reach a System Central Line-associated Bloodstream Infection Standardized Infection Ratio target of 0.7, or a reduction of 30 percent.
Assist International (New York, U.S.)
Assist International (AI), a non-profit building humanitarian programs in more than 60 countries, commits to partnering to implement safe drinking water, sanitation, and hygiene (WASH) programs, including water filtration systems and technical services, at health facilities in low- and middle-income countries. AI commits to prioritizing WASH as a core pillar of its mission and advocating for better WASH in healthcare facilities with partners, including governments. AI will also use lessons learned from the 52 sustainable Safe Water programs already implemented in partnership with GE Foundation and Emory University to better assess WASH in facilities and document implementation and evaluation methods.
Asolva, Inc. (California, U.S.)
Having served as IT professionals in healthcare for nearly 20 years, Asolva, Inc. commits to work with public health agencies to enable antibiotic use reporting at any health care facility regardless of size, geography, infrastructure, and resources. Asolva aims to partner with state health departments to expand antibiotic use reporting to CDC’s National Healthcare Safety Network with the use of their reporting technology that will work with any hospital for a low cost.
Association for Professionals in Infection Control and Epidemiology (Virginia, U.S.)
The Association for Professionals in Infection Control and Epidemiology (APIC) will work with its more than 15,000 members that save lives in hospitals and other healthcare settings to: increase clinician education and training through updated online and in-person educational programs, a joint position paper between APIC, SHEA, and SIDP, and a new chapter on antimicrobial stewardship in the APIC Text Online; conduct legislative and regulatory advocacy in support of funding for infection prevention and antimicrobial resistance programs; and develop consumer information and education on the proper use of antibiotics.
Association of Public Health Laboratories (Maryland, U.S.) , Association of State and Territorial Health Officials (Virginia, U.S.), Council of State and Territorial Epidemiologists (Georgia, U.S)
The Association of Public Health Laboratories (APHL), the Association of State and Territorial Health Officials (ASTHO), and the Council of State and Territorial Epidemiologists (CSTE) commit to engaging with chief health officials, state laboratory directors, and state epidemiologists from all 50 states and the territories to identify state-level commitments in support of the AMR Challenge. Over the next year, we will work with states, territories, and local health departments to identify areas for progress related to antimicrobial resistance across laboratory capacity, infection prevention and control, and antibiotic use. Knowing that resistance does not respect geopolitical borders, these state commitments will collectively lead to a healthier and safer U.S.
Aster DM Healthcare (India)
Aster DM Healthcare—an integrated healthcare service organization with 20 hospitals, 112 clinics, and 213 pharmacies across nine countries—commits to standardization of reporting of multi-drug resistant organisms (MDRO) across the chain of hospitals of Aster DM Group in India, and regular monitoring of MDRO rates across these hospitals with a data review every three months. Aster also commits to regularly monitor and report on the consumption of antibiotics in the World Health Organization’s list of reserve antibiotics, and to proactively reduce consumption of these drugs by at least 20 percent by December 2020. Aster will also pursue the use of molecular diagnostics for rapid and accurate identification of resistance threats, and will review the diagnostics’ utility in rationalizing antibiotic use and reducing consumption.
AtlantiCare Health System (New Jersey, U.S.)
AtlantiCare Health System, a member of Geisinger Health System that includes two hospitals and the AtlantiCare Physician Group with 5,800 employees and more than 900 physicians at nearly 100 locations in Southern New Jersey, commits to decreasing the spread of infection through prevention such as improved hand washing and increased vaccine utilization for AtlantiCare’s employees, patients, and the community. To contribute to solving the problem of inappropriate prescribing and drug resistant organism development, AtlantiCare commits to making hand washing a system wide goal, reminding all patients and employees to wash appropriately to minimize infection. AtlantiCare is also educating patients and staff about antibiotic resistance and appropriate prescribing of antibiotics, and undergoing a rigorous flu vaccination campaign for the community.
Atrium Health (North Carolina, U.S.)
Atrium Health will work with its more than 900 care locations and 44 hospitals to use Targeted Assessment for Prevention (TAP) strategies to reduce the Standardized Infection Ratio by a minimum of 10 percent by 2020. Atrium Health will also increase the number of its acute inpatient facilities that submit antibiotic use data to the National Healthcare Safety Network to 50 percent by 2021. Atrium Health will reduce inappropriate antibiotic prescribing by 10 percent by 2020 through the Atrium Health Outpatient Antimicrobial Stewardship program. Globally, Atrium Health will continue partnerships with two hospitals in Guatemala by assisting them in developing recommendations, educational plans, and facility specific policies to reduce antibiotic resistance.
Banka BioLoo Limited (India)
Banka BioLoo Limited, an organization in India that promotes and develops environmentally-friendly products and services for human waste management, commits to implementing safe drinking water, sanitation, and hygiene (WASH) in 50 healthcare facilities in India where WASH facilities are not currently available, beginning in 2020. In partnership with other organizations, Banka BioLoo Limited will also conduct WASH awareness programs in healthcare facilities selected by partner organizations. Progress will be measured through an annual review of healthcare facilities provided with WASH facilities.
Better Life for All Foundation (Nigeria)
The Better Life for All Foundation (BLAF), a non-governmental organization in Nigeria, commits to sustaining grassroots education and enlightenment on the use and misuse of antibiotics while promoting awareness on the global public health threats of antibiotic resistance. Having successfully led several grassroots awareness on the dangers of antibiotics misuse and antimicrobial resistance (AMR), BLAF will continue to reach out to adolescents, youths and nursing mothers with sensitization programs to promote infection prevention and control and antibiotics stewardship. Through community outreach in the coming year, BLAF will advocate for social and behavioral changes that promote antibiotic stewardship and infection prevention and control.
BD (California, U.S.)
BD (Becton, Dickinson and Company) commits to helping slow the spread of antibiotic resistance by improving awareness, surveillance, infection prevention, and stewardship. BD is mobilizing the Antimicrobial Resistance Fighter Coalition campaign to improve awareness; will support surveillance and research by collating, in specific projects, MedMinedTM data with the CDC to help better understand resistance in the U.S. and track regional differences in important resistant pathogens and antimicrobial use; is advancing infection prevention practices by introducing innovative infection control solutions and deploying training programs in collaboration with international organizations and professional societies; and is helping extend the useful life of existing medications through stewardship training and innovative integration of diagnostic testing, microbiology results and medication management workflows.
Bill & Melinda Gates Foundation (Washington, U.S.)
The Bill & Melinda Gates Foundation has always prioritized the fight against drug resistance in our infectious disease work, from TB, HIV, diarrheal diseases and malaria to, increasingly, pneumonia and neonatal sepsis. Together with our partners, we will continue to support research and development of vaccines and novel biologics to prevent drug-resistant diseases and protect lives, especially in low- and middle-income countries. We are also committed to investing in research to understand the burden of antimicrobial resistance on vulnerable populations in order to better quantify the impact of vaccines on drug-resistant infections and inform innovative approaches to preventing resistant infections.
BIOCOM AG (Germany)
BIOCOM AG commits to establishing a long-term international networking platform through its annual Novel Antimicrobials and Antimicrobial Resistance (AMR) Diagnostics conference occurring in Berlin in March 2019. The platform will connect small and medium enterprises in Europe that are driven by research and development and focused on AMR with pharmaceutical companies, academia, investors and governmental institutions across the globe. This will allow small and medium enterprises to discuss their specific challenges in bringing new antimicrobial treatments and diagnostics to the market. This will include global networking with a focus on AMR innovation, financing, and investments between AMR innovators and investors as well as between human and animal health stakeholders.
bioMérieux (France)
bioMérieux, one of the largest in vitro diagnostics companies focusing on infectious diseases, is taking a comprehensive approach to detect, identify, monitor, track, and prevent antibiotic resistance, while also working with agricultural, veterinary, and pharmaceutical companies and various healthcare providers to limit resistance development and maximize the utility of existing and in-development antibiotics. With approximately 75 percent of bioMérieux’s 2018 clinical research and development budget dedicated to products that support the fight against antibiotic resistance, bioMérieux commits to developing assays and systems that allow the rapid and focused choice of the most appropriate antibiotic(s) in order to reduce inappropriate antibiotic use, overall antibiotic administration, and to diminish the global threat of antibiotic-resistant infections. bioMérieux also commits to reducing antibiotic resistance through appropriate patient prescribing, limiting antibiotics in animals, the identification of foodborne pathogens, and the safety of pharmaceutical compounds.
BIOMIN (Austria)
BIOMIN, an animal nutrition firm that harnesses the power of science to support farm animal health and performance naturally, commits to expanding the reduction of non-medically necessary use of antibiotic feed solutions across the agriculture sector globally. The BIOMIN Research Center undertakes considerable scientific research and development to further enhance the sustainability of the livestock and aquaculture industries and to limit or decrease rates of antibiotic resistance on farms. BIOMIN will continue to support this initiative through knowledge sharing of scientific findings and industry best practices along with technical consulting for commercial partners.
Bio-Rad Laboratories (California, U.S.)
Bio-Rad Laboratories commits to positively impacting appropriate and targeted use of antibiotic prescriptions by advancing diagnostic technologies that enable rapid identification of bacteria resistant to antibiotics from the current standard of 24 to 48 hours to less than an hour. Bio-Rad’s technology developments, to be brought to market by 2020, will enable doctors to prescribe the correct treatment for patients much faster and will help reduce antibiotic resistance by limiting unnecessary exposure to antibiotics.
Biotia (New York, U.S.)
Biotia, an artificial intelligence, health tech firm, commits to advancing available technology such as sequencing-based laboratory workflow, software, and databases to help quickly and accurately identify and track antimicrobial resistance in healthcare settings. Biotia will conduct at least three pilots in hospitals to test viability and implementation of AMR products, as well as collect substantial amounts of DNA sequence data of pathogens and resistant germs. These data will build a database to inform work in clinical settings and prevent infections. Biotia will also share the results of this work through at least one peer-reviewed manuscript, the press, scientific meetings, and with Biotia hospital customers.
BioVersys (Switzerland)
BioVersys AG, a Swiss pharmaceutical company, commits to initiating clinical trials in 2020 for their advanced research and development programs for hospital infections and tuberculosis. BioVersys AG focuses on research and development of small molecules acting on novel resistant bacterial targets and targeted microbiome therapies.
BJC Healthcare and Washington University School of Medicine (Missouri, U.S.)
BJC HealthCare, a system of 12 hospitals in Missouri and Illinois, and Washington University School of Medicine in St. Louis commit to improving penicillin allergy documentation by 20% in BJC facilities by the end of 2019. According to CDC, 10% of the U.S. population reports a penicillin allergy but less than 1% of the whole population is truly allergic. Broad-spectrum antibiotics are often used as an alternative to penicillin, driving antibiotic resistance. BJC HealthCare and Washington University School of Medicine will develop and implement a penicillin allergy assessment and testing protocol across all BJC facilities by the end of 2019. This assessment will improve antibiotic use in BJC facilities by allowing for the safest and most effective antibiotics to be prescribed.
Boehringer Ingelheim (Germany)
Boehringer Ingelheim, a company focused on advanced disease prevention for animal health, commits to reducing the use of and need for antibiotics in animals through an integrated health management approach using technologies that enable effective monitoring, early detection, and accurate diagnosis of resistant infections in animals. As part of the company’s annual training programs in 2019 and beyond, more than 1,000 professionals will be trained on disease control, judicious use of antibiotics, and other topics related to animal health. Boehringer Ingelheim will continue to find novel and innovative solutions to animal health issues, focusing on preventing and managing diseases by investing approximately 9% of the company’s net sales into research and development for paraciticides, vaccines, live biotherapeutics, diagnostics, and monitoring.
Bolb Incorporated (California, U.S.)
Bolb Incorporated is a manufacturer of germicidal Ultraviolet C Light Emitting Diode (UVC LED) platforms for chemical-free and touch-free disinfection of hospital environments to prevent exposure to infectious pathogens. Bolb commits to performing three hospital user studies during 2020 to measure the impact of its novel solutions and report results in infection prevention journals. The use of UVC in healthcare facilities is regulated by the Environmental Protection Agency and is currently deployed in more than 500 hospitals globally. The wider deployment of new LED capabilities has the potential to protect patients, nurses and workers, improving outcomes, and, as a result, lowering reliance on the use of antibiotics.
Boston Scientific (Massachusetts, U.S.)
Boston Scientific, a manufacturer of medical devices used in interventional medical specialties, commits to developing new configurations of their EndoKit™, designed to provide components needed to comply with industry guidelines, mitigate cross-contamination risk, and reduce variability during endoscope reprocessing, by the end of 2019. Boston Scientific also commits to providing and expanding their single-use products designed to minimize the risk of infection transmission and improve efficiencies by eliminating the need for reprocessing and tracking.
Boulos & Cooper Pharmaceuticals (Australia)
Boulos & Cooper Pharmaceuticals commits to developing a new class of antibiotics that has shown activity against all drug-resistant bacteria by investing in research and development of a new mechanism of action. Additionally, Ramizol®, a drug for C. difficile, is at pre-clinical development and will commence its first clinical trials on human subjects in 2020.
Bugworks Research, Inc. (India)
Bugworks, a drug discovery company that aims to discover novel pharmaceutical assets for combating AMR, will remain heavily invested into the R&D of novel broad-spectrum antibiotics that will be able to handle the worst global superbugs and be able to save many lives all over the world. We will continue to endeavor to beat superbugs via innovation, while making these products accessible and available to humanity. Bugworks will work closely with the government of India and other countries to promote stewardship of these novel antibiotics, so that these drugs stay effective for many years.
Burkina Faso Observatory for Quality and Safety of Care (Burkina Faso)
Burkina Faso Observatory for Quality and Safety of Care (OBQUASS) commits to partnering with non-governmental and governmental organizations to strengthen safe drinking water, sanitation, and hygiene (WASH) interventions in healthcare facilities. OBQUASS commits to monitoring all regions of Burkina Faso for improvement of WASH conditions in healthcare facilities and sending documented WASH reports to health authorities. OBQUASS will also conduct a national survey of healthcare facilities in its partner network to gather information on the state of WASH services in Burkina Faso and advocate for WASH services in their respective facilities.
C Diff Foundation (Florida, U.S.)
C Diff Foundation commits to continuing to recognize the serious disease burden and significant economic impact that antimicrobial resistance (AMR) and healthcare-associated infections place on patients, their families, in communities, and the health care systems throughout the world. As a part of the AMR Challenge, the C Diff Foundation will continue to acknowledge and share the importance of AMR stewardship programs; develop or expand ongoing campaigns at national or sub-national levels to promote and improve hand hygiene methods and compliance among health care providers; and make reliable and validated information available on antibiotic use and AMR stewardship programs, infection prevention, environment safety, and vaccines, therapeutics and diagnostics at the community and district levels to encourage best-practices.
California Department of Public Health (California, U.S.)
The California Department of Public Health (CDPH) commits to addressing the threat of antimicrobial resistance through a coordinated department-wide strategic framework for preventing the emergence and containing the spread of antimicrobial resistance in California. In 2018, the CDPH Healthcare Associated Infections (HAI) Program and local public health staff recruited the clinical laboratory that serves 12 of 22 California long-term acute care hospitals to conduct enhanced surveillance and confirmation of Candida auris. The CDPH HAI Program has responded to four high-concern antimicrobial resistance mechanisms detected by six California laboratories recruited to participate in the Antibiotic Resistance Laboratory Network targeted surveillance program.
Capital Health (New Jersey, U.S.)
Capital Health, a two-hospital health system serving 83,737 covered lives in New Jersey, commits to a collaborative approach to meet challenges posed by antibiotic resistance. The hospitals’ Antimicrobial Stewardship Program Committee and the Infection Prevention Committee will work hand-in-hand to reduce inappropriate use of antibiotics and adverse events, as well as increase infection prevention interventions such as vaccination initiatives and averting hospital-acquired infections. The 2019 goals include a 10% decrease in fluoroquinolone and aztreonam usage in patients with a history of penicillin allergy and 20% decrease in hospital-onset C. difficile infections.
CARB-X (Massachusetts , U.S.)
CARB-X will invest $80 million globally by December 2019 to support more than 40 antibiotic resistance product developers worldwide, including at least 10 new classes against Gram-negative bacteria, five new diagnostics, and 10 new non-traditional alternatives or vaccines. Given the importance of supporting the proper use of these lifesaving drugs when they are needed most, each award agreement will include commitments to access and stewardship of these new antibiotics, vaccines, and diagnostics.
CarePortMD, LLC (Delaware, U.S.)
CarePortMD, a platform that connects patients with clinicians in Delaware and Pennsylvania, commits to evidence-based prescribing of antibiotics. CarePortMD will implement a process for educating patients at the time of withholding antibiotics coupled with a protocol for scheduling a brief telemedical follow up assessment within 24-72 hours. This prescribing process provides patients and prescribers with either reassurance regarding the treatment plan, or an opportunity to modify use if a primary bacterial or emerging superinfection is identified.
Catholic Relief Services (Maryland, U.S.)
Catholic Relief Services (CRS), a non-profit helping people in emergencies earn a living through agriculture and access to affordable health care, commits to spreading United States Agency for International Development (USAID’s) Clean Clinic Model to strengthen health systems so that facilities, districts, provinces, and regions can identify, prioritize, implement, and fundraise for safe drinking water, sanitation, and hygiene (WASH). From 2019-2024, CRS will direct interventions at more than 300 healthcare facilities. CRS commits to influence national health and water policies and budgets in each country to replicate the Clean Clinic Model across territories, effectively improving WASH in healthcare facilities across every facility.
CDC Foundation (Georgia, U.S.)
The CDC Foundation is honored to support the U.S. Government’s AMR Challenge. We are committed to mobilizing resources in support of CDC’s work to combat antibiotic resistance through the development of public-private partnerships, creating opportunities for donors—individuals, corporations, foundations and organizations—to contribute, in response to the yearlong AMR Challenge and beyond. Together with our Board of Directors and Corporate Roundtable on Global Health Threats, the CDC Foundation stands with CDC and partners across sectors and around the world to join in the fight against the global threat of AMR. We believe that together our impact is greater.
Centers for Disease Control and Prevention (Atlanta, U.S.)
CDC is committed to providing the evidence base to identify the most effective safe drinking water, sanitation, and hygiene (WASH) and infection prevention and control (IPC) interventions in healthcare facilities. CDC is also committed to providing technical assistance to partners to implement those interventions. CDC strengthens capacity through training and developing global guidance documents used worldwide. CDC will continue these activities to support partners, help ensure sustainability, and integrate WASH in healthcare facilities into larger health initiatives as resources permit. CDC is committed, through CDC’s AMR Challenge, to engage stakeholders to address WASH and IPC capacity across settings, sectors, and countries.
Center for Global Safe WASH at Emory University (Atlanta, U.S.)
The Center for Global Safe Drinking Water, Sanitation, and Hygiene (WASH) at Emory University commits to the application of rigorous scientific methods to address the most pressing WASH research questions in healthcare facilities, such as determining the health outcomes of mothers and neonates who receive care from healthcare facilities that have safe or unsafe WASH conditions. Emory commits to the continued dissemination of information and maintaining a web-based platform that facilitates connection, the sharing of best practices, and promotion of sustainable interventions among a community of practice of more than 600 individuals from non-governmental organizations, government, engineering, and private corporations.
Center for Integrated Management of Antimicrobial Resistance (Massachusetts, U.S.)
The Center for Integrated Management of Antimicrobial Resistance (CIMAR), a collaboration between Tufts University and Tufts Medical Center, commits to develop combination drug therapies for human and animal medicine that treat infections and slow antimicrobial resistance. CIMAR engages in environmental surveillance in healthcare, soil, and water to control the spread of antimicrobial resistance, using tools from epidemiology, engineering, and the social sciences. Scientists find levels of resistance all over the country and map them to determine where the sources are located. CIMAR also commits to promoting the responsible use of antibiotics to improve patient treatment outcomes and is developing high school and undergraduate-level educational programs about antibiotic resistance.
Cepheid (California, U.S.)
Rapid and accurate diagnostic tests can help healthcare providers improve antibiotic prescribing decisions. Cepheid commits to develop and market molecular diagnostic products to optimize antimicrobial use in patients; provide diagnostic tests and disease tracking software that facilitates public health surveillance of infectious organisms, allowing for real-time detection of the organisms and drug resistance; collaborate directly with pharmaceutical companies to develop tests that support clinical trials of new antibiotics; and work with academic, industry, and government partners to develop sound public policy regarding the promotion of antimicrobial infection control and antibiotic stewardship programs.
Chicago Area Patient-Centered Outcomes Research Network (Illinois, U.S.)
The Chicago Area Patient-Centered Outcomes Research Network (CAPriCORN) is a partnership of research institutions, clinicians, patients, and patient advocates working to improve health care quality, health outcomes, and health equity. CAPriCORN institutions will improve the tracking of inpatient antibiotic use and resistance by generating measures using a common data model from PCORnet, a national research data network. These measures will be made available to local antibiotic stewardship and infection control programs, and shared across CAPriCORN sites to improve antibiotic use and slow spread of resistant infections.
Chicago Department of Public Health (Illinois, U.S.)
As a local health department responsible for direct healthcare facility engagement, the Chicago Department of Public Health (CDPH) commits to continued surveillance and response to antibiotic-resistant infections in healthcare settings. CDPH conducts onsite investigations and advises providers on infection control gap mitigation. CDPH will leverage surveillance data for public health response and train healthcare staff across the care continuum in core antibiotic stewardship principles and appropriate antibiotic use. CDPH commits to working with state, local, academic, and clinical partners to establish regional standards for infection control and antibiotic stewardship by 2020 to reduce emergence and spread of multi-drug resistant bacteria.
Chicago Prevention and Intervention Epicenter (Illinois, U.S.)
The Chicago Prevention and Intervention Epicenter will reduce “nightmare bacteria” carbapenem-resistant Enterobacterales (CRE) in Chicago by July 2019. In collaboration with state and local public health departments, Rush will use regional strategies and work with state and local partners to prevent CRE infections and protect the Chicago metropolitan region of 9.5 million from its spread. The Epicenter will also work through the CDC-funded Chicago PROTECT project to improve interfacility communication and reduce spread between high-risk facilities.
Christian Health Association of Ghana (Ghana)
Christian Health Organization of Ghana (CHAG) commits to adopting safe drinking water, sanitation, and hygiene (WASH) as a vital tool for reigniting primary healthcare as a means of attaining universal health coverage for all in Ghana, including rural and underserved areas. CHAG further commits to integrate WASH in all services it provides, including health training schools. CHAG’s role as key champions, pioneers, and advocates of WASH will be a priority commitment until all CHAG facilities become fully WASH compliant by the year 2030.
Circle of Life Healthcare Private Limited (India)
Circle of Life Healthcare Private Limited, a healthcare analytics company in India, commits to continue developing antimicrobial stewardship software using artificial intelligence technology to deliver a personalized predicted antibiogram for patients, allowing prescribers to prescribe more accurately while waiting on culture results from laboratory tests. The software has been deployed in some large tertiary care hospitals in the Indian subcontinent with expansion plans to Singapore and the United States. Circle of Life is measuring the software’s effectiveness by improved patient outcomes and reduced length of hospital stays.
CityMD (New York, U.S.)
CityMD Urgent Care—a healthcare company that operates more than 100 urgent care centers in New York, New Jersey, and Washington State—commits to partnering with CDC, the Antibiotic Resistance Action Center (ARAC), and the Urgent Care Association of America (UCA) for antibiotic stewardship initiatives. Along with these partner organizations, CityMD will promote education, adoption, and implementation of toolkits, and tracking of stewardship metrics by measuring and tracking the Healthcare Effectiveness Data and Information Set (HEDIS) measures for the diagnoses of pharyngitis, bronchitis, and upper respiratory infection. CityMD is committed to improving antibiotic stewardship across the urgent care industry and promoting the highest level of care for the communities they serve.
Cleveland Clinic Abu Dhabi (United Arab Emirates)
Cleveland Clinic Abu Dhabi commits to improving antibiotic use and reducing hospital-onset C. difficile infection and multi-drug resistant organism rates through continued implementation of their successful advanced antibiotic stewardship program (ASP). The ASP was found to be associated with a decrease in antibiotic use. Cleveland Clinic Abu Dhabi’s ASP is focused on raising awareness of optimal usage of antibiotics. The program’s effectiveness has been measured by tracking antimicrobial consumption (i.e., days of therapy per 1000 patient days), measuring infections due to hospital-acquired multi-drug resistant organisms including C. difficile infections, and tracking ASP team interventions performed on monthly basis and antimicrobial cost.
Clinical & Laboratory Standards Institute (Pennsylvania, U.S.)
The Clinical & Laboratory Standards Institute (CLSI) is a global non-profit organization that develops medical laboratory testing standards based on input from and consensus among industry, government, and health care professionals. Through CLSI’s Subcommittee on Antimicrobial Susceptibility Testing (AST), which provides useful information to laboratorians to assist clinicians in the selection of appropriate antimicrobial therapy for patient care, CLSI collects and reviews AST data from a variety of sources and studies. Using this data, CLSI commits to developing AST standard reference methods; providing AST testing quality control parameters; establishing breakpoints and epidemiological cutoff values; and providing information on testing and reporting. In January 2020, CLSI will publish the 30th edition of M100, a publication that will contain current information for drug selection, interpretation, and quality control.
Clinique Naoufel (Algeria)
Clinique Naoufel, a private hospital in Algeria, commits to improve antibiotic use and reduce the spread of resistant infections by implementing best practices recommended by the CDC for antibiotic stewardship and infection prevention and control throughout its facility.
Clorox Healthcare (California, U.S.)
In 2019, Clorox Healthcare commits to improving infection prevention practices through presentations at the scientific meetings of the Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Association for Professionals in Infection Control and Epidemiology, and Association for the Health Care Environment; publications in peer-reviewed journals like American Journal of Infection Control and Infection Control & Hospital Epidemiology; and peer-to-peer sharing to add to the knowledge base that informs infection prevention and control guidelines and practices implemented in healthcare facilities every day. Clorox Healthcare will also continuously innovate to make effective infection prevention products, and support front-line healthcare and environmental services professionals with evidenced-based protocols, education, and training. Finally, Clorox Healthcare commits to raising awareness of infection prevention and stewardship priorities among healthcare providers and the general public.
Coalition for Improving Sepsis and Antibiotic Practices (U.S.)
The Coalition for Improving Sepsis and Antibiotic Practices—made up by medical diagnostics companies including Thermo Fisher Scientific, Roche Diagnostics, bioMérieux, and Abbott—commit to international efforts to improve sepsis care, promote antibiotic stewardship, and improve patient health outcomes. These companies, employees, and partner organizations commit to advancing knowledge among clinicians, policymakers, and payers of the benefits of using innovative, biomarker-assisted sepsis treatment and antibiotic use to improve critical public health outcomes.
Colorado Department of Public Health and Environment (Colorado, U.S.)
The Colorado Department of Public Health and Environment (CDPHE) commits to addressing the threat of antibiotic resistance by creating a comprehensive state report that will be publicly available on the CDPHE website by December 2, 2019. The report will include pathogen-specific information, and act as an educational resource to empower community members and stakeholders to better understand and respond to resistance threats in Colorado. CDPHE will support a coordinated and collaborative response to antibiotic resistance in Colorado. CDPHE will continue to monitor antibiotic-resistant pathogens through surveillance, and update the report in 3 years.
Conduent (New Jersey, U.S.)
Conduent will provide an IT solution that facilitates infection surveillance and antibiotic use data sharing. The Midas Cloud collects and analyzes vaccine, diagnostic, and therapeutic data that can help track antimicrobial resistance (AMR) trends, drive decisions, and accelerate progress against AMR. Conduent is committed to making rapid software enhancements to the Midas Cloud, which can accept data from a number of systems, as AMR threats and priorities emerge.
The Department of Public Health from Connecticut (Connecticut, U.S.)
Connecticut Department of Public Health (CT DPH) commits to expanding capacity within Connecticut to detect, prevent, and respond to antimicrobial resistance. CT DPH will continue to respond to novel and targeted multidrug-resistant organisms according to CDC guidance, with the assistance of advanced molecular detection at the CT State Public Health Laboratory and CDC’s Antibiotic Resistance Lab Network. CT DPH also commits to supporting antibiotic stewardship activities across the state using updated educational materials on antibiotic resistance and appropriate antibiotic use. CT DPH is also a CDC Emerging Infections Program site with special projects in the areas of methicillin-resistant Staphylococcus aureus, carbapenem-resistant organisms, and multidrug-resistant foodborne disease attribution.
ContraFect Corporation (New York, U.S.)
ContraFect Corporation, a developer of biologic therapies, commits to developing new treatment options for life-threatening, antibiotic-resistant infectious diseases using direct lytic agents (DLAs) from its lysin and amurin product platforms. Lysins are a new therapeutic class of proteins that kills bacteria, eliminates biofilms, and positively interacts with conventional antibiotics. Amurins are a new class of antimicrobial peptides which exhibit broad-spectrum activity against a wide range of antibiotic-resistant Gram-negative pathogens, including Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacter species. These new therapeutic modalities could provide meaningful improvements to clinical outcomes for patients with antibiotic-resistant infections.
ConvenientMD Urgent Care (New Hampshire, U.S.)
ConvenientMD Urgent Care commits to educating its providers and patients on the importance of antimicrobial stewardship, in conjunction with providing high quality, evidence based medical care to our communities. ConvenientMD is now working on a study aimed at decreasing the use of unnecessary antibiotics in patients who present with acute viral pharyngitis. ConvenientMD’s goal is to decrease antibiotic prescribing in its busy, on-demand urgent care clinics while maintaining and/or improving patient satisfaction. ConvenientMD anticipates improving provider satisfaction by supplying evidence-based guidelines and patient education materials.
Council for Outbreak Response: Healthcare-Associated Infections and Antimicrobial-Infections and Antimicrobial-Resistant Pathogens (United States)
The Council for Outbreak Response: Healthcare-Associated Infections (HAIs) and Antimicrobial-Resistant (AR) Pathogens (CORHA) commits to improving practices and policies for detecting, reporting, investigating, and controlling HAI/AR outbreaks. CORHA commits to publishing and promoting thresholds, outbreak definitions, and investigation tools for pathogens such as carbapenem-resistant Enterobacterales and Candida auris. In addition, CORHA commits to providing a forum for healthcare and public health partners to work together to improve communication such as patient notification and laboratory practices in support of HAI/AR response activities.
CVS Health (Rhode Island, U.S.)
MinuteClinic, the retail medical clinic of CVS Health and largest provider of retail health care in the U.S., will continue to provide evidence-based and high-quality care of infectious diseases, using rigorous guidelines to support antibiotic prescribing decisions. By June 2019, MinuteClinic will advance efforts to educate patients about the appropriate use of antibiotics, and will support providers by providing the tools necessary to be antimicrobial stewardship leaders. Antibiotic prescribing will continue to be a key component of MinuteClinic’s clinical quality review process, to include safety reviews, ongoing performance measurement, and national benchmarking.
DC Health (Washington, DC, U.S.)
DC Health, the District of Columbia’s health department, commits to making antibiotic resistance data more available to local antimicrobial stewardship and infection control experts working in DC healthcare facilities. DC Health will create public dashboards and written reports that share data generated from CDC’s Antibiotic Resistance Laboratory Network, CDC’s National Healthcare Safety Network, and healthcare facility antibiograms. DC Health will consult with the Healthcare-Associated Infections Advisory Committee to create reporting methods to ensure usability and sustainability. DC Health will continue using local antibiotic resistance data to alert individual healthcare facilities of emerging threats to take appropriate actions to prevent spread.
Delaware Division of Public Health (Delaware, U.S.)
Delaware Division of Public Health commits to a coordinated statewide approach promoting the appropriate use of antibiotics, improving patient outcomes, and decreasing the spread of multidrug-resistant pathogens. The Choosing Wisely statewide antibiotic stewardship initiative reiterates that not all infections warrant antibiotics and seeks to ensure that right antibiotics are given for the right clinical conditions, at the appropriate doses and durations. Tactics include the reduction of inappropriate antibiotics for viral upper respiratory infections in the outpatient setting through provider education and of the use of empiric antibiotics beyond 72 hours in hospitalized patients suspected of having an infection.
Desert Research Institute and Transform International (Nevada, U.S.)
Desert Research Institute (DRI) and Transform International (TI) are organizations investigating the effects of natural and human-induced environmental change and building local capacity in developing countries. The organizations commit to developing frameworks for long-term sustainability of safe drinking water, sanitation, and hygiene (WASH) services in healthcare facilities throughout the developing world by focusing on strengthening local capacity, training, monitoring, evaluation, resolution, and learning. DRI and TI aim to develop, test, and share guidelines that facility staff and local governments can use, providing a system of ongoing operation and maintenance of WASH services.
Development Associates, LLC (Maryland, U.S.)
Development Associates LLC—a global health consulting company—commits to monitoring and tracking antimicrobial resistance (AMR) in Latin America and the Caribbean to provide information for medical research developments and reduce the burden of disease on communities. Development Associates will use existing data from peer review journals and research studies and conduct focus groups to develop community interventions to track and monitor AMR. Success will be measured by a reduction of 35% in AMR infections over time.
DNV GL Healthcare (Norway)
DNV GL Healthcare (DNV GL) provides healthcare accreditation and program certification services to more than 500 healthcare organizations in the U.S. DNV GL has implemented or will implement antibiotic stewardship standards for DNV GL accredited acute care and critical access hospitals by the close of 2019. DNV GL has also established a certification in infection prevention that includes antibiotic stewardship requirements, and encouraged all DNV GL accredited organizations to participate in the AMR Challenge. DNV GL will also invite DNV GL accredited and certified organizations to participate in a forum on the AMR Challenge as part of the 2019 Healthcare Symposium.
Doctor on Demand (California, U.S.)
Doctor On Demand is a national virtual medical care practice providing video-based encounters across all 50 states 24/7/365 days a year, delivering one million face-to-face doctor visits addressing urgent issues, chronic care conditions like hypertension and mental health. Across its hundreds of providers, Doctor on Demand will lower unnecessary antibiotic prescribing by 20 percent by December 2019 for acute upper respiratory infections including bronchitis, sinusitis and pharyngitis and require two hours of continuing education training for all providers.
Duke University and the Duke Center for Antimicrobial Stewardship and Infection Prevention
(North Carolina, U.S.)
Duke University and the Duke Center for Antimicrobial Stewardship and Infection Prevention commits to working with colleagues in 20 hospitals throughout the southeastern U.S. to implement the CDC toolkit for C. difficile prevention, including interventions to improve tracking of infection, infection prevention interventions such as hand hygiene and contact precautions, environmental disinfection, and antimicrobial use such as decreased use of fluoroquinolones.
E-Clinic (Mongolia)
E-Clinic—an integrated healthcare platform company with more than 200 healthcare organizations in Mongolia—commits to developing antimicrobial stewardship software to facilitate more accurate prescriptions by clinicians. E-Clinic also commits to sharing data and best practices with local health providers to improve use of antibiotics and infection prevention and control.
Ecolab, Inc. (Minnesota, U.S.)
Ecolab provides water, hygiene, and energy technologies and services at nearly 3 million locations in more than 170 countries. Ecolab commits to researching customer food safety and public health risk management by staying current on emerging trends; optimizing Ecolab’s response to emerging research and insights in line with the risks presented; and communicating these findings to promote balanced, risk-based approaches to combating antibiotic resistance through hygiene and sanitation program implementation at scientific and trade conferences and in publications.
ECRI Institute (Pennsylvania, U.S.)
ECRI Institute is an independent non-profit organization that advances effective, evidence-based health care. In 2019, ECRI commits to conducting hands-on evaluation and testing of infection reduction technologies, such as hand hygiene monitoring systems, ultraviolet disinfection systems, and disinfection caps. ECRI will also analyze reports of adverse events and near misses shared by healthcare delivery organizations, develop strategies for reducing harm, vet the trustworthiness of guidelines, and disseminate guidance and alerts that prioritize patient safety and strategies for infection control and prevention.
Ecumenical Pharmaceutical Network (Kenya)
Ecumenical Pharmaceutical Network (EPN), a network of 118 members in 37 countries in Africa, commits to conducting activities to combat antibiotic resistance through Church Health Institutions (CHIs). EPN will raise awareness about the problem of antibiotic resistance; promote infection prevention programs including safe drinking water, sanitation, and hygiene (WASH); promote antibiotic stewardship programs; and work to increase access to quality-assured antibiotics. EPN commits to incorporating WASH into materials, trainings, and advocacy within the CHIs. During its contributions at the regional level with WHO, CDC Africa, and other intergovernmental organizations, EPN will contribute and support the global WASH agenda and advocacy.
Elanco (Indiana, U.S.)
Elanco, an animal health company dedicated to developing innovative solutions that protect and enhance animal health, commits to investing at least 50 percent of their food animal research and development budget in projects that will lead to alternatives for medically important antibiotics, including introduction of non-medically important antibiotics to replace colistin use in key emerging markets; engaging in two partnerships to increase access to veterinary and professional oversight in countries with limited resources; improving responsible antibiotic use by expanding data collection and analytics of animal husbandry and health outcomes to inform best practices; and by encouraging implementation of vaccination and nutrition programs to prevent disease.
Elemeno Health (California, U.S.)
Elemeno Health, a cloud-based solution for engaging front-line healthcare teams, commits to continued promotion of antibiotic stewardship and the prevention of healthcare-associated infections through microlearning tools such as interactive guides, smart checklists, and concise how-to videos. These tools currently are accessible and actionable in 11 healthcare organizations in the U.S. anytime and anywhere.
Emory Antibiotic Resistance Center (Georgia, U.S.)
The Emory Antibiotic Resistance Center (ARC) commits to hosting interdisciplinary brainstorming and networking sessions to discuss cutting edge research findings and collaborative research opportunities in conjunction with ARC seminars given by invited external experts three times each year. These sessions will feature a seminar presented by a national expert on antibiotic resistance. Participants across the leading research institutions in Georgia, including Georgia Institute of Technology, University of Georgia, Morehouse School of Medicine, and Emory University will share research findings and promote the use of shared infrastructure and collaborations across institutions.
ENABLE (Belgium)
ENABLE, an antibacterial drug discovery engine funded by Innovative Medicines Initiative (IMI), commits to developing novel antibiotic compounds targeting Gram-negative bacteria by investing about €19 million in research and development in 2019 and until January 2020. By 2020, ENABLE commits to increasing the pipeline of medicines against Gram-negative infections including: three antibacterial lead compounds, two antibacterial development candidate medications, and at least one compound for preclinical and Phase 1 clinical studies.
Engineering Ministries International (Colorado, U.S.)
Engineering Ministries International (EMI), a Christian non-profit development organization of architects, engineers, surveyors, and construction managers, commits to being a technical resource for the design and implementation of appropriate and sustainable safe drinking water, sanitation, and hygiene (WASH) solutions in the healthcare facilities and communities where they work. With offices in 10 countries and projects in more than 90 countries, EMI works within the local context to design and construct culturally-appropriate facilities that are sustainable, affordable, and transformational.
European Centre for Disease Prevention and Control (Sweden)
The European Centre for Disease Prevention and Control (ECDC), an European Union (EU) agency dedicated to strengthening Europe’s defenses against threats such as antimicrobial resistance, commits to working with EU member states, other agencies and stakeholders across surveillance, epidemic intelligence, evidence-based guidance and systematic reviews, training, and support. ECDC launched an online resources directory to support EU member states and institutions in developing guidelines for the prevention and control of antimicrobial resistance and healthcare-associated infections. Since 2008, ECDC also coordinates European Antibiotic Awareness Day, raising awareness about the need for prudent use of antibiotics and will hold one on November 18, 2019.
Evolve Biosystems, Inc. (California, U.S.)
Evolve Biosystems commits to providing infant focused microbiome-based solutions to aid in the fight against antimicrobial resistance, including diagnostic tools, under the Evivo brand. Evolve is currently preparing for a clinical study to start late 2019 to test a point of care device that screens infant stool for abundance of potential pathogens known to harbor resistance genes. Evolve is building a database with collaborators aimed at creating and disseminating a deeper, more robust understanding of the infant gut metagenome, how it differs from adults, how exposure and diet shape this community, and the abundance of antimicrobial resistance within infants and children globally in different healthcare settings.
Express Scripts (Missouri, U.S.)
Express Scripts is the largest independent manager of pharmacy benefits in the U.S. and one of the country’s largest pharmacies. Serving more than 85 million people, Express Scripts commits to leverage prescription drug administrative claims for insights on clinicians prescribing pattern.
FAIRR Initiative (United Kingdom)
The Coller FAIRR Protein Producer Index is a comprehensive assessment of how publicly-listed, global producers of meat, dairy, and fish are managing sustainability risks, including antibiotic use on farm animals. FAIRR commits to using evidence from the Index to engage the world’s largest animal protein producers and consumers on better antibiotic stewardship practices. This commitment will enable FAIRR to drive changes in corporate practice to reduce and ultimately phase out the routine use of antibiotics to protect public health and optimize their long-term value. Company progress will be assessed on an annual basis. FAIRR is a global network of institutional investors focused on building a sustainable food system that is supported by more than 200 investors representing $12 trillion in combined assets.
Faiths for Safe Water (New York, U.S.)
Recognizing that increasing WASH in healthcare is vital to combating antimicrobial resistance, Faiths for Safe Water (FSW), an advocacy project prioritizing funding for the global water crisis, offers pro bono media consulting to multi-faith leaders and faith-based organizations making a commitment to safe drinking water, sanitation, and hygiene (WASH) in healthcare facilities. FSW will assist in strategically editing and placing content such as blogs, op-eds, and select video through cultivated, targeted media opportunities. FSW will perform content editing and ongoing placement. In addition, FSW invites clergy to its informal board of advisors to increase reach and impact of WASH advocacy.
Government of Finland (Finland)
The Finland Ministry of Social Affairs and Health, National Institute for Health and Welfare (THL), Ministry of Agriculture and Forestry (MMM), and Finnish Food Safety Authority (Evira) commit to developing a real-time notification and reporting system to detect unusual events, including extremely antimicrobial-resistant bacteria, at local, regional, or national level to support outbreak investigations and infection control measures; improving surveillance of human antimicrobial consumption; constructing a system to collect use data on all animal species; and establishing an expert group on antimicrobial resistance (AMR) and antimicrobial consumption in each hospital that follows and controls AMR infections and steers the use of antimicrobials.
First Arabian Drug Information Center (Saudi Arabia)
The First Arabian Drug Information Center (FADIC), a professional health care organization in the Middle East, commits to maintaining the appropriate use of antibiotics through antimicrobial stewardship education and by providing certification in antimicrobial stewardship training programs for pharmacists, physicians, nurses, and other healthcare professionals. FADIC will also provide a roadmap for applying antimicrobial stewardship in multiple healthcare settings, including acute-care hospitals, long-term care facilities, ambulatory care settings, and community pharmacies to provide an evidence-based practice. In addition to antimicrobial stewardship, FADIC will research, consult, and promote awareness.
First Light Diagnostics (Massachusetts, U.S.)
First Light Diagnostics, Inc., a developer of diagnostic tests, commits to delivering a platform in 2020 that will enable clinicians to treat patients with targeted antibiotics at the onset of multi-drug resistant infections. First Light Diagnostics will manage the testing and reporting processes using a user-friendly interface or have the option to integrate with a hospital laboratory information system to provide clinicians with diagnostic testing results. The platform will help eliminate the unnecessary use and overuse of antibiotics that contribute to the rise of antibiotic-resistant pathogens by providing the care team with information to inform diagnosis.
Florida Department of Health (Florida, U.S.)
The Florida Department of Health (Florida Health) commits to promoting evidence-based strategies for reducing the spread of antibiotic-resistant organisms, improving prescribing practices with data sharing among health care personnel, and containing outbreaks of novel organisms. Florida Health will continue working with health care facilities to offer educational sessions, on-site assessments, and outbreak assistance. Florida Health will also develop and provide health care facilities with antimicrobial stewardship toolkits, a standardized process for conducting infection control assessments, and a tracking system for outbreak responses.
Foundation for Innovative New Diagnostics (Switzerland)
The Foundation for Innovative New Diagnostics (FIND), working on the development and delivery of diagnostics to address global health priorities, commits to developing new tests by 2021 to definitively diagnose gonorrhea prior to treating patients with antibiotics. FIND commits to developing one or more targeted sequencing solutions for the diagnosis of drug-resistant tuberculosis. FIND is also creating digital solutions to link antimicrobial resistance (AMR) data from local sources, such as clinics and laboratories, to national-level surveillance systems. Its AMR Diagnostics Use Accelerator evaluates a package of diagnostic, clinical algorithms, and behavior change interventions. FIND is providing evidence to inform policy change at the country level that can positively impact AMR.
Foundation for Neglected Disease Research (India)
Foundation for Neglected Disease Research (FNDR), a research organization based in India, commits to delivering novel drug candidates for various neglected diseases and developing inexpensive, quick, and accurate diagnostic methods. FNDR currently has two drug candidates for drug-resistant tuberculosis (TB), one which is partnered with the TB Alliance, and the other is a proprietary molecule in a late stage preclinical development that could be released in the next 5-7 years.
Government of France
France commits to continued implementation of its Interministerial Roadmap for Controlling Antimicrobial Resistance (AMR). This One Health focused action plan includes 40 actions across five objectives: raising awareness among the public and healthcare professionals; antibiotic stewardship and infection prevention and control; research and innovation; monitoring and surveillance and governance; and intersectoral policy. The plan coordinates ministries, agencies, and research organizations at a national level. France will continue to make strides in these areas and will measure progress using a set of indicators. France is also coordinating the European Union Joint Action on AMR and Healthcare-Associated Infections (EU-JAMRAI), scheduled for August 2020 with a plan for sustainability and integration of concrete measures into national action plans.
Gelest, Inc. (Pennsylvania, U.S.)
Gelest, Inc., a manufacturer of silicone and silanes for healthcare products, commits to improving the sanitation of public facilities such as hospitals, hospitality and food service establishments, and airports by preventing the spread of harmful germs and antibiotic-resistant strains. Gelest, Inc. will develop sanitation and infection prevention products that reduce the excessive use of antibiotics in humans to treat and prevent infections. The products will include antimicrobial laundry additives, antimicrobial surface cleaning wipes, and antimicrobial wound care dressings, and will launch in 2019-2021.
Genesis Healthcare, Inc. (California, U.S.)
Genesis Healthcare, Inc., one of the nation’s largest post-acute care providers, commits to developing best practices for enhanced contact precautions to decrease transmission of novel multi-drug resistant organisms (MDROs) in all of its skilled nursing facilities. These precautions require healthcare facility personnel to wear a gown and gloves during high-contact, resident care activities to assist in reducing the spread of targeted MDROs. Success will be measured through adherence to precautions and reduced numbers of targeted MDROs in healthcare acquired infections, hospitalizations and antibiotic use. The results will be shared publicly through communication with other long-term care providers and professional organizations.
Georgia Department of Public Health (Georgia, U.S.)
The Georgia Department of Public Health (GDPH) commits to using survey data from 350 dentists in the state to improve antibiotic use in dental settings. GDPH will also use state hospital data collected by CDC’s National Healthcare Safety Network to create benchmarks on appropriate antibiotic prescribing for patients in Georgia and surrounding U.S. southeast states.
Global Antibiotic Research and Development Partnership (Switzerland)
The Global Antibiotic Research and Development Partnership (GARDP), a non-profit public health innovator, commits to delivering five new antibiotic treatments by 2025 in response to the growing burden of antibiotic-resistant infections. These treatments focus on the priority pathogens identified by the World Health Organization, current unmet needs for diseases and key populations—including treatments for serious bacterial infections in adults and children, newborns with sepsis, and for sexually-transmitted infections. GARDP believes all infections should be treatable and aims to make the treatments it develops affordable and accessible to people who need them, wherever they are in the world.
Global Handwashing Partnership (Washington, D.C., U.S.)
Global Handwashing Partnership (GHP), a coalition of partners that promote handwashing at local, national and international levels, commits to advocating for improvements in safe drinking water, sanitation, and hygiene (WASH), particularly hand hygiene in healthcare settings globally. GHP will reach out to private entities, academic institutions, multilateral and government agencies, and non-governmental and community-based organizations to encourage action on hand hygiene; profile global success stories; compile and disseminate key evidence; and equip advocates with tools and data to impact handwashing among target populations. GHP will also coordinate hand hygiene advocacy efforts to help members across sectors become hand hygiene advocates.
Global Health Alliance Melbourne (Australia)
Trends project that by 2050, Asia will have the highest number of deaths attributable to antimicrobial resistance (AMR) on earth. Through 36 member organizations across nine sectoral types, the Global Health Alliance Melbourne (GLHAM) commits to a collective and coordinated multidisciplinary approach to addressing AMR at a national and regional level. GLHAM will take joint action through its AMR community of practice to strengthen regional health security through diagnosis and measurement; preventing transmission of existing resistant strains; and preventing emergence of new resistant strains. GLHAM’s membership ranges from nongovernmental to hospitals, universities, and the private sector.
Global Health Council (Washington, D.C., U.S.)
Global Health Council (GHC), a global membership organization supporting and connecting global health advocates worldwide, commits to advocating with the U.S. government and global organizations for policy and programs that lead to improvements in safe drinking water, sanitation, and hygiene (WASH) in healthcare facilities. GHC will convene and connect non-governmental organizations, private sector, and academia that work in global health and WASH to help coordinate efforts to address WASH in healthcare facilities.
Global Health Technologies Coalition (Washington, D.C., U.S.)
The Global Health Technologies Coalition (GHTC), a coalition of more than 30 nonprofit organizations, academic institutions, and aligned businesses, commits to encouraging leadership among our member organizations in developing new diagnostics, vaccines, and therapeutics to combat antimicrobial resistance (AMR). To spur research and development of new antibiotics and other technologies to detect, prevent, and treat resistant pathogens, GHTC will continue to advocate for strengthened funding, new policies, frameworks, and incentive mechanisms, and enhanced coordination. In particular, we will continue to advocate for the Global Health Security Agenda to embrace and support R&D for technologies to combat AMR in its 2019-2024 framework.
GoHealth Urgent Care (Georgia, U.S.)
GoHealth Urgent Care and Northwell Health are proud to work directly with the CDC and Antibiotic Resistance Action Center (ARAC) on antibiotic stewardship initiatives, and to serve as leaders and champions for antibiotic stewardship in urgent care. One measure of our collective success will be the adoption and practical use of antibiotic stewardship, the CDC’s antibiotic stewardship core elements, and the MITIGATE toolkit in the adult and pediatric emergency and urgent care settings. The ultimate goal of our commitment is to improve overall antibiotic stewardship both in urgent care and more broadly across other ambulatory care settings.
Harvard Medical School: Program in Global Surgery and Social Change (Massachusetts, U.S.)
The Program in Global Surgery and Social Change at Harvard Medical School commits to fighting antimicrobial resistance globally. Serving as a technical partner to ministries of health, it develops, finances, and implements their national programs for the strengthening of emergency and essential surgical care and anesthesia. The Program in Global Surgery and Social Change commits to promoting policies to strengthen data collection of surgical infections, to reduce the rate of surgical infections, and to encourage responsible antibiotic use.
Hawaii Department of Health (Hawaii, U.S.)
Public health officials from Hawaii commit to addressing the threat of antibiotic resistance by educating healthcare providers on antibiotic stewardship, increasing laboratory capacity to detect antibiotic resistance mechanisms, rapidly implementing containment response measures, and preventing infections in Hawaii’s residents.
Healthcare Association of New York State (New York, U.S.)
The Healthcare Association of New York State (HANYS) provides leadership, representation, and service to more than 400-member healthcare organizations throughout New York. HANYS commits to supporting healthcare organizations as they accelerate their focus on improving antibiotic use. Additionally, HANYS commits to continue working to ensure every New York resident has access to affordable, high-quality care. Its Antibiotic Stewardship Collaborative helps healthcare organizations improve their antibiotic stewardship efforts and prepare for anticipated mandates. Through the Collaborative, HANYS members have access to regular educational sessions, consultation with state and national experts, networking opportunities, and facility-specific antibiotic utilization reports with statewide comparisons.
HealthforAnimals (Belgium)
HealthforAnimals, the global association representing animal medicine companies and regional/national industry associations, commits to addressing antibiotic resistance by supporting One Health approaches that work across human and animal health; encouraging responsible antibiotic use of antibiotics; promoting disease prevention and increased access to products and expertise for farmers, veterinarians, and pet owners; investing in development of products for prevention and treatment such as vaccines, diagnostics, nutritional products, and more; and increasing knowledge, transparency, and communication within the industry. HealthforAnimals and members will take action on this commitment through training, new investments in research and development, value-chain collaborations, and more.
HCA Healthcare (Tennessee, U.S.)
HCA Healthcare includes 178 hospitals and 119 surgery centers in the U.S. that together provide more than 28 million patient encounters each year. HCA will continue to measure environmental cleaning to help reduce the spread of resistant germs in healthcare facilities; work with CDC to drive use of the days of therapy (DOT) and Standardized Antimicrobial Administration Ratio (SAAR) benchmarking data; and continue to participate in public health research that uncovers new ways to protect people from AMR. HCA will also ensure all of its hospitals enroll in the National Healthcare Safety Network Antibiotic Use and Resistance data reporting modules, and ensure that all HCA hospitals work with local and state public health to implement CDC’s containment strategy.
Henry Ford Health System (Michigan, U.S.)
Henry Ford Health System commits to improve antibiotic use and reduce the spread of resistance across the continuum of patient care in southeast Michigan and among our global partners. Henry Ford will expand current efforts for global antibiotic stewardship and infection control education and mentoring in Michigan, educate patients about appropriate antibiotic use in urgent care and community settings, and align antibiotic stewardship interventions with priorities and strategies of national and international organizations.
HP, Inc. (California, U.S.)
HP, Inc. is committed to making its D300e Digital Dispenser, an inkjet technology that can automate and miniaturize dispensing of antibiotics for rapid susceptibility testing, available to CDC’s Antibiotic Resistance Laboratory Network to help fight antibiotic resistance. By continuing work with CDC on their pilot and working with public health labs in the U.S. and worldwide, HP hopes to enable rapid reference method testing of new antibiotics for resistant infections. HP is committed to continuing to refine the HP D300e Digital Dispenser system by seeking feedback from partners to address this growing public health need. HP will also provide system and application support to public health labs working to validate and use the system in their laboratories.
Hy-Plains Feedyard, LLC (Kansas, U.S.)
Hy-Plains’ Education Research Center, a commercial cattle feeding operation in southwest Kansas, commits to identifying methods to affect and potentially slow the development of antibiotic resistance in the feedyard industry. Hy-Plains has the ability to conduct studies in real-world settings to evaluate new products or practices with the ability to scale it through the industry. Hy-Plains has conducted vaccine, feed additive, antibiotic treatment, immune response, and retrospective data analysis trials and commits to working with partners to bring the results of its research to scale throughout the industry in a more rapid manner
IAVI (New York, U.S.)
IAVI, a global organization to accelerate the development of vaccines, commits to the discovery, development, and delivery of affordable antibodies for sustainable access to patients all over the world, including low- and middle-income countries. IAVI will work with global policymakers, health care providers, manufacturers, and funders through its landscape assessment project on global access for antibody-based therapies and prevention. IAVI will work with these partners to build innovative business models enabling sustainable, global access to vaccines for drug-resistant infections and other diseases.
Department of Health and Welfare from Idaho (Idaho, U.S.)
The Healthcare Associated Infections (HAI) Program within the Department of Health and Welfare from Idaho commits to addressing the threat of antimicrobial resistance through collaboration with state partners and Idaho’s healthcare facilities to support infection prevention efforts and improve the use of antibiotics throughout the state. Specifically, the HAI Program supports Idaho’s hospitals, long-term care facilities, and outpatient clinics in an effort to improve antibiotic use and provide opportunities for education on appropriate antimicrobial use and resistance to healthcare workers and/or patients.
Illinois Department of Public Health (Illinois, U.S.)
The Illinois Department of Public Health commits to continued collaboration with partners to implement and refine the Illinois Action Plan to Prevent Healthcare Associated Infections and the Illinois Multi-Drug Resistant Organism Surveillance Investigation and Response Plan. The department coordinates a tiered, regional response to identify affected patients, provide technical assistance to facilities on infection prevention and control practices, and improve communication between facilities by reporting antimicrobial-resistant pathogens and sending automated alerts. The department will also continue to support antimicrobial stewardship by offering resources and technical assistance to healthcare facilities and prescribers to ensure antibiotics are appropriately used to treat infections.
Illumina (California, U.S.)
Illumina, a global genomics company, commits to support a meeting in collaboration with the American Society for Microbiology to influence change in policy, education and health outcomes associated with antibiotic resistance. Bringing together international thought leaders in policy, healthcare, environment, and veterinary medicine, Illumina’s goal is to impact diagnostic and therapeutic approaches and policies to combat the development and spread of antibiotic resistance, to preserve vital antibiotics for future generations. Illumina is also committing to provide early access for novel applications to U.S. CDC laboratories and researchers to spearhead evaluation of these applications and to determine their applicability to enhance programs to combat antibiotic resistance.
ILÚM Health Solutions (New Jersey, U.S.)
ILÚM, a provider of technology and services to support infectious disease management, commits to continue bringing modern technology to healthcare settings domestically and, in partnership with the CDC, to low- and middle-income countries to improve the way patients are cared for. ILÚM recognizes that health systems often struggle to associate individual clinical decisions to long-term outcomes, such as antimicrobial resistance, and are often limited by connectivity of electronic resources. This commitment is carried out through patient and population care tools, such as ILÚM Insight, that combine clinical decision support, data science, molecular diagnostics, and robust care tracking and reporting into vertically integrated systems that improve population health.
ImmunoBiology Limited (United Kingdom)
ImmunoBiology Limited—a technology company in the United Kingdom developing vaccines for infectious diseases—commits to developing a universal multi-protein pneumococcal vaccine for global use to fight against antibiotic resistance. Vaccines used for pneumococcal disease are a long-term solution to the problem of antibiotic resistance. Unfortunately, current carbohydrate vaccines only cover a limited number of circulating pneumococcal strains. Universal multi-protein pneumococcal vaccines can be used widely in both the developed and developing world and contribute to the fight against pneumococcal antibiotic resistance.
Government of India – Department of Biotechnology, Ministry of Science and Technology (India)
The Department of Biotechnology (DBT) leads the biotechnology policy, strategy, centers, and programs related to biotechnology in India. As part of its Mission Program on Antimicrobial Resistance, DBT commits to collaborating with DBT’s Biotechnology Research Assistance Council (BIRAC) to launch a Joint Call for Innovative Approaches to address antimicrobial resistance (AMR) in October 2018, with a focus on developing new antibiotics and antibiotic alternatives. DBT also commits to fostering a network of researchers, product developers, and incubators that can work together on AMR, and will explore the possibility of establishing an Indo-US mobility fellowship in AMR that could fund research exchanges under Indo-US collaborative initiatives. In order to catalyze the scientific and technological innovation necessary to address AMR, BIRAC commits to collaborating with the charity, Nesta, to support a Longitude Prize to develop point-of-care diagnostic tests to identify AMR pathogens.
Government of India – Indian Council of Medical Research, Ministry of Health and Family Welfare (India)
The Indian Council on Medical Research, the country’s premier medical research institute and one of the oldest medical research bodies in the world, is committed to: supporting basic and clinical research; working with the Indian Council of Agriculture and Research (ICAR) to create a One Health platform for integrated surveillance; strengthening, standardizing and expanding capacity of the public health and healthcare systems in India to implement appropriate infection control procedures; enhancing stewardship practices and strengthening hospital-based surveillance for HAIs, including working with regulatory bodies to address the excess and access issues; and collaborating with international partners, including the U.S. CDC, the U.S. National Institute for Allergy and Infectious Diseases (NIAID), Research Council Norway, and the German Federal Ministry of Education and Research (BMBF) to develop a better understanding of resistance mechanisms and discover new drug targets and drug molecules.
Indiana Department of Health (Indiana, U.S.)
The Indiana Department of Health commits to addressing the threat of antibiotic resistance through data-driven education for healthcare facilities, providers, and patients in the state. Indiana Department of Health will provide onsite education and infection control assessments to combat novel and pan-resistant organisms and will include recommendations for appropriate antibiotic resistance screenings, trainings on antibiotic resistant threats, and public resources.
Infectious Disease Society of America (Virginia, U.S.)
Infectious diseases physicians are at the forefront of local, national, and international efforts to address antimicrobial resistance (AMR). These providers lead infection prevention and stewardship programs; conduct basic, translational, and clinical research; advance public health interventions; and diagnose and treat patients infected by multidrug resistant pathogens. In 2019, the Infectious Disease Society of America will invest $5 million in educational efforts on AMR, $500,000 in AMR policy and advocacy efforts, and $400,000 in AMR communications.
Institute of Neurosciences Kolkata (India)
The Institute of Neurosciences, a hospital in Kolkata, Eastern India that treats neurological disorders and reaches 300 million people in the region and neighboring areas, commits to the implementation of proper hand hygiene to combat the spread of germs by avoiding handshakes for all staffs and healthcare providers.
Intermountain Healthcare (Utah, U.S.)
Intermountain Healthcare—which includes 22 hospitals and more than 185 clinics in Utah and Idaho—will launch a patient-centered initiative targeting outpatient antibiotic prescribing. The initiative will include novel antibiotic prescribing metrics with prescriber feedback, electronic health record tools, education for patients and providers, and a public awareness campaign. Intermountain will also launch the Healthy Patient Initiative to build on current infection prevention activities. This includes improving patient hygiene, environmental hygiene, and caregiver infection prevention strategies. The initiative will promote oral care and bathing, hand hygiene, and bundle compliant device maintenance.
International Association of Plumbing and Mechanical Officials and LIXIL Americas (California, U.S.)
The International Association of Plumbing and Mechanical Officials (IAPMO) and LIXIL Americas commit to working with other industry stakeholders to strengthen the governance, skilled labor, product development, and supply chain sourcing necessary to make safe drinking water, sanitation, and hygiene (WASH) services sustainable. In 2019, the collaborative will host a convening involving key stakeholders from the plumbing industry, governments, non-government organizations, and multilateral institutions in a targeted session on WASH in healthcare facilities. The collaborative also commits to working with a host community in carrying out a demonstration project facilitating the development of WASH services in an underdeveloped healthcare facility.
International Centre for Antimicrobial Resistance Solutions (Denmark)
International Centre for Antimicrobial Resistance Solutions (ICARS), an international One Health knowledge and applied research partnership, commits to generating, assessing, and using evidence to mitigate antimicrobial resistance (AMR), focusing on low and middle-income countries. ICARS will work with countries’ priorities to translate national action plans and policies into evidence-based practices on the ground, while building capacity and capability within countries. It will aim to partner with public, academic, philanthropic, and private sectors to combine resources for the development of solutions supporting the global effort to tackle AMR.
International Confederation of Midwives (Netherlands)
The International Confederation of Midwives (ICM) commits to incorporating safe drinking water, sanitation, and hygiene (WASH) advocacy into communications and advocacy plans and activities. ICM will promote adequate availability and use of WASH in healthcare facilities worldwide through advocacy and outreach to ICM’s network of stakeholders. ICM commits to recognize the role of WASH as fundamental to the delivery of quality care and to communicate this message as part of ICM’s mission to strengthen the midwifery profession globally.
International Consortium for Antimicrobial Stewardship in Agriculture (Washington, D.C.)
The International Consortium for Antimicrobial Stewardship in Agriculture (ICASA) —one of the largest public-private partnerships focusing on improving antibiotic use (antibiotic stewardship) in animal agriculture—commits to investing in research to accelerate innovation and antibiotic stewardship across the livestock supply chain. ICASA participants will work collaboratively to improve the health and welfare of beef cattle, pigs, and poultry through the development of practical solutions, including advanced tools and management practices to address the underlying drivers of antibiotic use in livestock. By leveraging knowledge and resources from diverse organizations, ICASA will advance stewardship and improve health outcomes for livestock.
International Livestock Research Institute (India)
The International Livestock Research Institute (ILRI) is a non-profit organization that focuses on value chains, health-environment linkages, and knowledge and research on livestock. As part of the AMR Challenge, ILRI commits to: providing guidance on best practices for preventing the release of antibiotics into the environment; advocating for good stewardship of antibiotics in the livestock sector, including the restriction of antibiotics for growth promotion and pre-mixed feed; and promoting a One Health approach to better understand the human-animal-environment relationship of antimicrobial resistance.
IntraHealth International (North Carolina, U.S.)
IntraHealth International, a non-profit to improve the performance of health workers in developing countries, commits to elevating the expertise of frontline health workers, especially from low- and middle-income countries, in policymaking discussions on safe drinking water, sanitation, and hygiene (WASH) in health facilities. IntraHealth International will advocate for global health policymakers and influencers to act on the acute effect that inadequate WASH has on health worker safety and their ability to carry out lifesaving work. IntraHealth International will continue to advocate for prominent placement of WASH in healthcare facilities on the agendas of health workforce and systems policy meetings.
Iowa Department of Public Health (Iowa, U.S.)
The Iowa Department of Public Health, the State Hygienic Laboratory, and the National Institute of Antimicrobial Resistance Research and Education commit to continuing to develop standardized case and outbreak investigation protocols for resistant organisms. Efforts will focus on collaborating with key partners—including professional boards, the state hygienic laboratory, and state universities—to continue containment efforts and identify new targets for public health prevention and interventions.
IQVIA (North Carolina, U.S.)
IQVIA is committed to identifying and understanding the driving factors behind the rising number of drug-resistant bacteria strains to inform ongoing prevention efforts. To improve antibiotic use, IQVIA is committed to building on an existing framework of antibiotic prescribing reporting and analysis. IQVIA will provide CDC with 50 state-specific reports featuring 2018 data. These data can be shared with individual states and advance CDC’s understanding of the dynamics behind overprescribing at the state and local levels. These reports will also contain urgent care facilities and their affiliated prescribers, to enable detailed analysis of provider prescribing patterns by CDC and the states across the range of acute care settings.
IRC (Netherlands)
IRC, a Dutch-based organization focused on systems approaches to achieve the Sustainable Development Goals, commits to advocacy for safe drinking water, sanitation, and hygiene (WASH) in healthcare facilities. IRC will bring civil society from the health and WASH sectors to national, regional, and global platforms to ensure WASH challenges and solutions reach key audiences. IRC will continue to support capacity development of civil society organizations at the country level to influence budgets and policies on WASH in healthcare facilities, specifically Ministries of Health. In Ghana and Uganda, IRC will partner to ensure that WASH in healthcare facilities in Asutifi North and Kabarole Districts meet national and global standards for basic services by 2030.
J. Arthur Dosher Memorial Hospital (North Carolina, U.S.)
J. Arthur Dosher Memorial Hospital, a critical access hospital in North Carolina, commits to reducing the inappropriate use of fluoroquinolones for urinary tract infections, community-acquired pneumonia, and skin infections by educating hospitalists and emergency department physicians. The goal is to reduce the inappropriate use of fluoroquinolones by 75% before June 30, 2019.
Jamii Medical Awareness (Tanzania)
Jamii Medical Awareness (JMA)—a Tanzanian non-profit promoting proper medication use, disease control measures, and sanitation—commits to providing educational outreach programs and workshops for community members and healthcare workers on the appropriate use of antibiotics and proper sanitation. JMA conducts several workshops and community outreach programs annually in health centers and schools on proper antibiotic use. JMA aims to bring behavioral changes for the appropriate use of antibiotics to improve the inappropriate use which accelerates the rise of antibiotic resistance. JMA will conduct surveys every quarter to assess the community’s level of knowledge and understanding of antibiotic resistance after providing services.
Government of Japan (Japan)
Japan commits to contribute to the establishment of an antimicrobial resistance (AMR) surveillance system in Asian countries where the status of AMR has not been systematically investigated by using the data integration system that the AMR Research Center has developed. Japan expects that this surveillance system, as well as provision of essential resources for the laboratory test such as devices and expertise, will greatly contribute to AMR investigations in Southeast Asia.
Joint Commission (Illinois, U.S.)
As an independent organization that accredits and certifies nearly 21,000 health care organizations and programs in the U.S., the Joint Commission will work to improve antibiotic use among its accredited organizations. The Joint Commission will implement antibiotic stewardship requirements in accredited hospitals, identify and share leading practices for antibiotic stewardship with organizations being surveyed, and develop new antibiotic use standards for ambulatory care providers by 2020.
Johnson & Johnson (New Jersey, U.S.)
Johnson & Johnson commits to developing and responsibly deploying innovative tools to combat antimicrobial resistance (AMR). Johnson & Johnson has one of the largest antimicrobial research and development pipelines, with a particular focus on multidrug-resistant tuberculosis (MDR-TB)—the leading contributor to AMR-related deaths—healthcare-associated infections, and more. Johnson & Johnson has committed to donating 105,000 courses of bedaquiline through 2019 via a four-year donation program, in partnership with United States Agency for International Development (USAID) and JSC Pharmstandard. Johnson & Johnson offers bedaquiline at a not-for-profit price to more than 130 countries via Stop TB Partnership’s Global Drug Facility.
Kaiser Permanente (California, U.S.)
As the largest interdisciplinary healthcare organization in the U.S., Kaiser Permanente commits to remaining a strong antibiotic steward and preventing the spread of resistant infections. Kaiser Permanente pledges to continue using data to avoid unnecessary antimicrobial use in our inpatient, outpatient, and skilled nursing facilities; improve infection prevention and control through the dedication to facilities’ sanitation, and environmental initiatives that include water quality management and thorough housekeeping and cleaning practices; and implement medical product sourcing processes in order to eliminate products with antimicrobial additives.
Kansas Department of Health and Environment (Kansas, U.S.)
The Kansas Department of Health and Environment commits to expanding its project with PipelineRx, a telepharmacy company. PipelineRx created a cloud-based integration platform that pulls data directly from electronic health records (EHR) and sends it to CDC’s National Healthcare Safety Network for upload into the Antimicrobial Use and Resistance (AUR) modules. The project will expand to include more facilities by creating integration platforms for the two most widely used EHR systems in Kansas by the end of 2020. This project will allow for an accurate representation of antibiotic prescribing and the epidemiology of resistant organisms.
Keep Antibiotics Working (Illinois, U.S.)
Keep Antibiotics Working, a coalition of 17 non-profit organizations, commits to ensuring that the sales of medically important antibiotics for use in livestock is reduced by 2021 by at least 45% from 2009 levels. Keep Antibiotics Working will work with all levels of U.S. government, livestock producers, supermarkets, restaurants, and other large purchasers of animal products
Kentucky Antibiotic Awareness (Kentucky, U.S.)
Kentucky Antibiotic Awareness (KAA) is a statewide campaign to reduce inappropriate antibiotic use. KAA commits to tracking antibiotic use in children using Medicaid claims data and sharing data analyses with state officials and healthcare providers. We also commit to providing resources and education for providers and patients and promoting collaboration among key stakeholders throughout the state.
Kentucky Department for Public Health (Kentucky, U.S.)
The Kentucky Department for Public Health (KDPH) commits to addressing the threat of antibiotic resistance through improving detection of resistance threats and strengthening capacity to respond and contain threats quickly. KDPH will improve detection through ongoing communication with infection preventionists and laboratories to increase knowledge and awareness of state reporting requirements, as well as recommended responses for identifying facilities. KDPH will build on relationships established with healthcare facilities, laboratories, and partner organizations to provide a coordinated approach to resistance threats. KDPH will measure progress by completeness of reporting and antibiotic resistance isolate submission.
Long Beach Department of Health and Human Services (California, U.S.)
California’s Long Beach Department of Health and Human Services is committed to improving surveillance of antibiotic resistance by hosting a carbapenem-resistant Enterobacterales (CRE) collaborative with the California Department of Public Health to create dialogue, education, and awareness among skilled nursing facilities and acute care hospitals. By December 2019, the Long Beach Department aims to have antibiotic resistance subject matter experts throughout many healthcare facilities. The Department made CRE reportable locally, and commits to finding new ways to detect outbreaks of resistant organisms more quickly and efficiently; ensure accurate and timely reporting of outbreaks of resistant organisms in local facilities; and monitor for novel multi-drug resistant organisms.
Louisiana Department of Health (Louisiana, U.S.)
The Louisiana Department of Health commits to addressing the threat of antibiotic resistance through detecting, containing, and responding to resistant organisms through laboratory surveillance, outbreak response, and improving antibiotic use across the provider spectrum. Additionally, public health epidemiologists at the Louisiana Department of Health commit to in-person and on-demand infection control and antibiotic stewardship education for providers. The health department will use provider-specific Core Elements for Antibiotic Stewardship education in its in-person and web-based trainings. The health department will target short-term and long-term acute care hospitals, inpatient rehabilitation facilities, and nursing homes.
Los Angeles County Department of Public Health (California, U.S.)
The Los Angeles County Department of Public Health (LACDPH) will continue to prevent, detect, and respond to antibiotic-resistant infections in healthcare settings, using surveillance data to identify threats requiring response. LACDPH will promote the appropriate use of antibiotics among the public and human and veterinary healthcare providers, and will train healthcare staff across the continuum of care in appropriate antibiotic use and stewardship.
Maine Department of Health and Human Services Center for Disease Control and Prevention (Maine, U.S.)
The Maine Department of Health and Human Services Center for Disease Control and Prevention commits to conducting surveillance for all recognized novel or targeted multidrug-resistant organisms (MDRO). The department will use CDC’s Antibiotic Resistance Laboratory Network to identify and confirm novel or targeted MDRO. It will work with healthcare facilities to determine if transmission of a novel or targeted MDRO has occurred when a patient with known infection has been admitted and help them identify and reduce infection control gaps. The department will offer education to infection preventionists on the occurrence of MDROs in the state and share lessons learned.
Making a Difference in Infectious Diseases (Connecticut, U.S.)
Making a Difference in Infectious Diseases (MAD-ID), a non-profit organization comprising pharmacists, physicians, nurses, and other health professions focused on continuing professional education in the general area of infectious diseases prevention and treatment and antimicrobial stewardship in particular, commits to optimizing antibiotic use in the practice community and thus reduce the prevalence and spread of resistance. MAD-ID will leverage innovative and practical educational and skills training in antibiotic stewardship and infectious diseases therapeutics. MAD-ID will also continue its support of research documenting effective interventions to provide an evidence base for these practices.
Management Sciences for Health (Massachusetts, U.S.)
Management Sciences for Health (MSH), a non-profit partnering to build strong and sustainable health systems, commits to increasing visibility of safe drinking water, sanitation, and hygiene (WASH) messaging in domestic and global health advocacy. MSH will work with technical experts to ensure WASH is accurately and adequately represented in global health security, including antibiotic resistance. MSH will work with 16 targeted countries to ensure that WASH is incorporated into health facilities during the next year. Through the Global Health Security Agenda Consortium, MSH will ensure these messages are raised to the Global Health Security Agenda Steering Group level through 2024.
Maryland Department of Health (Maryland, U.S.)
The Maryland Department of Health (MDH) commits to continued collaboration with healthcare facilities to implement CDC’s Core Elements of Antibiotic Stewardship in acute, long term, and ambulatory care settings. MDH will support appropriate use of antibiotics through the educational Campaign for Appropriate Antibiotic Use (CAAUse). Additionally, MDH is working with the local CDC Prevention Epicenters Program on the Statewide Prevention and Reduction of C. difficile Collaborative to assess hospitals with high C. difficile infection rates and help implement strategies to control the transmission of C. difficile in acute care hospitals. C. difficile rates are measured using National Healthcare Safety Network data.
The Massachusetts Department of Public Health (Massachusetts, U.S.)
The Massachusetts Department of Public Health (MDPH) commits to combating antibiotic resistance through core surveillance and coordinated prevention and control activities focused on multi-drug resistant organisms of concern. Current antibiotic stewardship efforts at MDPH include the analysis of statewide All Payer Claims Data to better understand the landscape of outpatient antibiotic use, including a focus on antibiotic use in dentistry. In addition, MDPH will use CDC’s National Healthcare Safety Network antibiotic use data from a number of participating Massachusetts acute care hospitals to develop facility-specific benchmarking and statewide analyses in 2019.
Max Healthcare (India)
Max Healthcare recognizes antimicrobial resistance (AMR) as a major concern threatening the safety and quality of the care it provides throughout the 14 hospitals in its system and the health outcomes of its patients. As one of India’s leading providers of comprehensive healthcare services, Max Healthcare commits to containing and preventing the spread of infections, particularly those that are resistant to antibiotics by: tracking infections via its AMR surveillance system and sharing data nationally as part of the Indian Council of Medical Research surveillance network; developing and implementing comprehensive policies on infection prevention and control that are in line with international standards from Joint Commission, CDC, and World Health Organization, but also incorporate local practice and context; and having five dedicated infection control nurses at the Max Super Specialty Hospital in Saket, a facility of 560 beds.
Mayo Clinic (Minnesota, U.S.)
Mayo Clinic commits to working with care providers and patients to implement interventions that enhance infection prevention and control measures, and to optimize antibiotic use while also increasing public awareness about antibiotic resistance. Improvements or a targeted 5 percent reduction in antibiotic use will be achieved by 2020. Mayo Clinic also commits to developing, sharing, and assessing the value of novel diagnostics in terms of patient outcomes and usage of antimicrobial agents for infectious diseases, which can help identify resistance and improve patient care.
McDonald’s Corporation (Illinois, U.S.)
McDonald’s Corporation commits to partnering with suppliers and producers on the responsible use of antibiotics in food animals in its supply chain, including a policy for chicken in the U.S. and markets around the world, and a newly launched antibiotic use policy for beef. As one of the world’s largest restaurant companies, McDonald’s aims to use its Scale for Good to help preserve the efficacy of antibiotics for future generations.
Medcare Hospital (United Arab Emirates)
Medcare Hospital—a 60-bed multi-specialty hospital in Dubai, United Arab Emirates—commits to implementing an antibiotic stewardship program in its facility. Medcare has implemented several strategies to support the appropriate use of antibiotics, such as developing an antibiotic stewardship working group to manage and monitor antibiotic usage. Program effectiveness is monitored by monthly tracking of antibiotic use and prescribing patterns in the hospital. Medcare aims for a 10% to 20% improvement in appropriate use of antibiotics in its facility by January 2020.
Medical University of South Carolina (South Carolina, U.S.)
The Medical University of South Carolina (MUSC) commits to advancing stewardship efforts by developing an evidence-based, institution-specific guidebook and mobile application. The MUSC Antimicrobial Stewardship Program is committed to expanding the hospital’s mission via innovative trainee education and optimization of antimicrobial stewardship protocols. Specifically, MUSC’s Stewardship Team will incorporate evidence-based practice and guidelines into an institutional guidebook for trainees via a mobile app. The team is also pioneering a decision-making tool for the electronic medical record system to promote successful stewardship efforts in conjunction with the Infection Control Team. This platform will streamline data collection and tracking of antibiotic use and infections.
MedLabs Diagnostics (New Jersey, U.S.)
MedLabs Diagnostics, a diagnostics testing laboratory, commits to innovating a rapid molecular diagnostic test that can genetically identify antibiotic resistance genes from pathogenic organisms with molecular stratification by the end of 2019. MedLabs Diagnostics aims for early detection of antibiotic resistance genes at the molecular genetics level that are emerging and evolving in the U.S. and the world, taking a novel molecular approach in promoting antibiotic stewardship and targeted guidance. With early detection of antimicrobial threats, MedLabs Diagnostics is making it possible to combat antimicrobial resistance with rapid detection diagnostics.
Medline Industries
Medline Industries, Inc.—manufacturer and distributor of medical products in more than 90 countries—commits to raising awareness of antibiotic resistance and combating emerging pathogens by supporting research and development aimed at reducing the spread of resistant pathogens. Medline will work with public health departments and academic centers across the United States to support industry and federal-funded clinical studies. This research will focus on preventing infections caused by multi-drug resistant organisms in high-risk patients in facilities such as intensive care units, wound infections with biofilm complications, post-discharge methicillin-resistant Staphylococcus aureus patients, and impact of environmental (e.g., room) cleaning protocols.
MEKS LifeHealth Initiative (Nigeria)
MEKS LifeHealth Initiative—a healthcare provider in Nigeria—commits to providing antimicrobial resistance (AMR) education to healthcare workers and local vendors during 2019 World Antimicrobial Resistance Awareness Day. MEKS also commits to providing handwashing training and awareness to medical students and schoolchildren. MEKS will partner with the Veterinary Virology Institute and Ministry of Environment to implement a One Health approach to combating AMR, recognizing that the health of people is connected to the health of animals and the environment. MEKS will collaborate with physicians and health professionals at the Jos University Teaching Hospital to implement an antimicrobial stewardship program.
Melinta Therapeutics, Inc. (Connecticut, U.S.)
Melinta Therapeutics, Inc., a commercial-stage company developing and bringing novel antibiotics to market to treat serious bacterial infections caused by antibiotic-resistant bacteria, commits to implementing comprehensive measures and metrics within the company’s commercial, manufacturing, and collaborative research initiatives to promote the responsible and sustainable use of its antibiotics as demonstrated by stewardship program implementation of early empiric therapy guidelines. Melinta’s education outreach program to improve antibiotic use will focus on unmet needs of clinicians participating in antibiotic stewardship programs in U.S.-based hospitals.
MeMed (Israel)
MeMed translates complex immune system signals into simple diagnostic insights to transform management of infectious disease—when and where needed. MeMed commits to providing a test addressing the daily clinical challenge that is a fundamental driver of antibiotic misuse—the difficulty in distinguishing between bacterial and viral infections. MeMed will collaborate with academic, commercial, and government partners towards the goal of making the test available to the broadest global population, as soon as possible. This new tool will provide actionable information to clinicians, promoting prudent antibiotic use and helping to tackle antimicrobial resistance.
Merck (New Jersey, U.S.)
New antibiotics are urgently needed to address growing antimicrobial resistance; however, there are relatively few in development. Merck, known as MSD outside the U.S. and Canada, is one of the remaining large pharmaceutical companies conducting research in this area. Merck commits to continuing to make significant investments in both early- and late-stage R&D to discover, develop, and commercialize novel vaccines and medicines to prevent and treat bacterial infections in both humans and animals. Merck focuses its research on pathogens found in CDC’s 2013 Antibiotic Resistance Threats Report and prioritized by the World Organization for Animal Health (OIE).
Michigan Department of Health and Human Services (Michigan, U.S.)
Michigan Department of Health and Human Services (MDHHS) commits to addressing antibiotic resistance through rapid detection, response, and containment. MDHHS will continue work with healthcare facilities and local health departments to report, respond, and contain the spread of carbapenemase‐producing carbapenem-resistant Enterobacterales (CP-CRE) to fulfill Michigan’s mandated reporting in the Communicable Disease Rules that were recently revised to include reporting for CP-CRE.
Micro-Scientific (Illinois, U.S.)
Micro-Scientific—a supplier of products used for the prevention of microbial transmission and cross-contamination—commits to developing two whitepapers about the dangers of ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa , and Enterobacter species) bacteria and how to protect patients and staff against these healthcare-associated infections. Micro-Scientific will also offer educational literature and presentations for the Association for Professionals in Infection Control and Epidemiology conferences focusing on education on ESKAPE pathogens and prevention of these multidrug-resistant organisms through effective environmental care.
MICROB-R (Chile)
MICROB-R, a multi-disciplinary research center in Chile studying antibiotic resistance, commits to educating the town of Molina, Chile on the appropriate use of antibiotics. In 2020, MICROB-R will show an educational video in waiting rooms of primary care facilities and will encourage health professionals working in those facilities to discuss the topic with patients. The campaign includes social media for further impact. MICROB-R aims to increase awareness in the Molina community and later expand the campaign to other Chilean communities. MICROB-R will survey an estimated 500 patients on the video content and their understanding of the responsible use of antibiotics.
Milken Institute (Washington, D.C., U.S.)
The Milken Institute is a nonprofit, nonpartisan think tank determined to increase global prosperity. We will highlight antimicrobial resistance (AMR) at our events around the world to foster greater awareness, connection, and urgency of the challenges of AMR among our network of tens of thousands of global leaders in business, government, science, technology, philanthropy, academia, media, and more. Through raising awareness, we aim to promote best practices and novel solutions, and advance biomedical research.
Millennium Water Alliance (Washington, D.C., U.S.)
Millennium Water Alliance (MWA), an alliance of safe drinking water, sanitation, and hygiene (WASH) charities in Asia, Africa and Latin America, commits to improving WASH in healthcare facilities in district-wide programming in northern Kenya. MWA will encourage members to incorporate WASH in healthcare facilities across all 90 countries where they operate, including coordinated country advocacy for healthcare facilities and sharing information with MWA members. MWA will support U.S. and East Africa advocacy for increased funding and prioritization for WASH, incorporating working in healthcare facilities as a key part of systems approaches and coordination with governments.
Minnesota Department of Health (Minnesota, U.S.)
Minnesota Department of Health (MDH) commits to expanding infection prevention and control efforts in healthcare facilities, with a particular focus on long-term care and ambulatory surgical facilities. MDH will also facilitate screening of high-risk patients upon admission for antibiotic-resistant threats to allow for more rapid detection and containment response efforts. In 2019, MDH will promote CDC’s National Healthcare Safety Network (NHSN) Antibiotic Use and Resistance Module for reporting of antibiotic use by hospitals within the state. By August 2020, MDH will summarize available (e.g., NHSN) and new data (e.g., setting-specific surveys) to describe awareness and impact of state stewardship initiatives and will engage with clinical partners to design new initiatives to advance stewardship in Minnesota outpatient and long-term care settings.
Minnesota One Health Antibiotic Stewardship Collaborative (Minnesota, U.S.)
The Minnesota One Health Antibiotic Stewardship Collaborative (MOHASC)—composed of more than 100 professionals including healthcare and animal health clinicians, industry professionals, academics, and state employees—commits to working together from 2019-2020 to develop clinical resources and tools to support antibiotic selection and tracking, implementation of CDC and American Veterinary Medical Association (AVMA) Core Elements of stewardship, and communication in medical and veterinary outpatient settings. MOHASC will apply evidence-based approaches to settings such as small animal and equine medicine where these types of data-driven resources for stewardship are lacking.
Mississippi State Department of Health (Mississippi, U.S.)
Mississippi State Department of Health commits to continued collaboration with the Mississippi Hospital Association (MHA) and the Mississippi Quality Innovation Network Quality Improvement Organization (MSQIN/QIO) to provide antibiotic stewardship education to healthcare providers to reduce inappropriate prescribing. Antibiotic stewardship education is provided through its ongoing partnership with MHA and MSQIN/QIO. Mississippi State Department of Health will also work with long-term acute care and long-term care healthcare facilities to perform two or more quarterly assessments of infection prevention and control practices, and promote proper infection prevention and control measures.
Missouri State Public Health Department (Missouri, U.S.)
The Missouri State Public Health Department (MSPHD) commits to addressing the threat of antibiotic resistance by continuing to test for carbapenem-resistant Enterobacterales bacteria throughout the state. The Missouri State Public Health Laboratory will also continue to send surveillance data to CDC’s National Antimicrobial Resistance Monitoring System for Enteric Bacteria, which provides information about emerging bacterial resistance and their spread.
Montana Department of Health and Human Services (Montana, U.S.)
The Montana Department of Health and Human Services commits to improving antibiotic use in outpatient settings among Medicare recipients by reducing use rates from 34 percent to 32.8 percent in 2019. Montana will continue its partnership with University of Montana Skaggs School of Pharmacy to implement antibiotic use tracking in 100 percent of acute care hospitals. Montana is implementing statewide interventions including clinical pathways for infections such as C. difficile, and systemic approaches based upon CDC’s Core Elements of Antibiotic Stewardship. This downloadable tool provides feedback to hospitals on antibiotic use. It also improves clinical laboratory result interpretation, accuracy of diagnosis and improved patient outcomes, and provider education and awareness of optimal antibiotic use.
Montefiore Medical Center (New York, U.S.)
Montefiore Medical Center is committed to improving antibiotic use through reporting and dissemination of antimicrobial utilization (AU) data and educating clinicians about appropriate antibiotic prescribing. Montefiore Medical Center will continue to submit AU data to CDC’s National Healthcare Safety Network and disseminate AU and Standardized Antimicrobial Administration Ratio benchmarking data to prescribers. Montefiore will also continue to educate medical students and residents through monthly seminars on antibiotic prescribing best practices, and a seminar in the spring of 2019 to educate medical students about appropriate antibiotic prescribing and the dangers of antimicrobial resistance.
Mountain Home Christian Clinic (Arkansas, U.S.)
Mountain Home Christian Clinic commits to giving clinic patients relevant educational materials regarding the proper use of antibiotics. To understand the impact of the materials, the clinic will track patients who receive the materials to identify if antibiotic treatment is initiated or not. Additionally, the in-clinic pharmacy is tracking how many antibiotics are filled per month and the resulting cost reduction (May 2019-May 2020) to monitor patient outcomes, further training of volunteer medical staff, and compare cost savings.
Mountain Safety Research Global Health (Washington, U.S.)
Mountain Safety Research (MSR) Global Health, a manufacturer of tools to improve drinking water and sanitation, commits to making safe drinking water, sanitation, and hygiene (WASH) in healthcare facilities a high-level priority in current and future work. MSR will look for opportunities to innovate new technologies and business models that support WASH in healthcare facilities and bring more attention to this critical issue. One such tool, the MSR SafiStation, produces sodium hypochlorite on site for infection prevention and control in health care facilities in low- and middle-income countries. MSR is currently testing the devices and hopes to have the product commercialized in 2020.
Mountaire Farms, Inc. (Delaware, U.S.)
Mountaire Farms, the seventh largest producer of chicken, commits to work together with other animal producers to establish a new One Health Certified (OHC) animal production standard open to any producer and across various commodity groups to be launched in 2019. This standard will be audited to certify compliance by the U.S. Department of Agriculture’s Agriculture Marketing Service with a goal to create a balanced, sustainable, and affordable cross-commodity, retail-labeled, animal-production standard with defined benchmarking requirements. One Health Certified meat products will be promoted directly to meat retailers, wholesalers, and restaurant chains and subsequently promoted by them to their respective customers.
National Association of County and City Health Officials (Washington, D.C., U.S.)
The National Association of County and City Health Officials (NACCHO) commits to increasing the awareness and capacity of the nearly 3,000 local health departments across the country to fight antibiotic resistance and promote antibiotic stewardship. NACCHO will facilitate opportunities for local health departments to identify local-level commitments in support of the AMR Challenge and share tools, resources, and lessons learned from local health departments related to AMR through the NACCHO website, blogs, and communications materials. NACCHO will continue to leverage its work with local health departments on healthcare-associated infections and with local STD clinics on resistant gonorrhea to advance detection, treatment, prevention, and containment of resistant infections.
National Association of Pediatric Nurse Practitioners (New York, U.S.)
National Association of Pediatric Nurse Practitioners (NAPNAP)—a professional pediatric nurse practitioner association with more than 9,000 members—commits to offering a pharmacology update intensive workshop annually at its national conference. NAPNAP has hosted two pharmacology update specialty symposia in various regions of the U.S. for the past five years. NAPNAP also offers this continuing education course online on its e-learning website. NAPNAP also commits to offering an antibiotic prescribing update and an antibiotic stewardship session.
National Cattlemen’s Beef Association (Colorado, U.S.)
The National Cattlemen’s Beef Association (NCBA) commits to the promotion of increased education concerning antimicrobial stewardship and the responsible use of antimicrobial drugs to prevent, control, and treat diseases in cattle, as guided by the national Beef Quality Assurance Program (BQA). By promoting increased participation and certification through BQA, NCBA seeks to increase awareness and compliance for responsible antimicrobial drug use in all segments of the beef cattle industry. Responsible use of antimicrobial drugs will aid in preserving the future effectiveness of antimicrobial agents against common pathogens in both human and animal species.
National Institute for Animal Agriculture (Colorado, U.S.)
The National Institute for Animal Agriculture (NIAA)— providing information, education, and solutions for 1,500 members across all animal agriculture industries—commits to educating animal agriculture professionals on responsible antibiotic use. NIAA will provide educational opportunities through its national symposia, website, social media, national news media releases, and partner organizations. NIAA will also provide partnership opportunities across the food supply chain to expand messaging. NIAA will focus on the evolving science of antimicrobial resistance at their annual antibiotic symposia in October 2019. NIAA commits to broadening best practices of responsible antibiotic use for better animal health and welfare, industry growth, and public trust.
National Institute for Antimicrobial Resistance Research and Education (Iowa, U.S.)
At the 2015 White House Stewardship Forum, the Association of Public and Land Grant Universities and the American Association of Veterinary Medical Colleges made a joint commitment to develop the National Institute of Antimicrobial Resistance Research and Education (NIAMRRE). NIAMRRE now commits to fostering the collaborative development of interdisciplinary research teams from diverse biologic, physical, and social science backgrounds. NIAMRRE commits to assisting these teams with developing research and educational projects that will address key knowledge gaps and develop practical interventions at the interface of agricultural, environmental, and human health. NIAMRRE consists of over 100 scientists representing all aspects of One Health working together to address critical antimicrobial resistance related issues.
National Milk Producers Federation (Virginia, U.S.)
The National Milk Producers Federation, which represents producers of the majority of the U.S. milk supply, commits to increase veterinary oversight of antibiotic use through the National Dairy Farmers Assuring Responsible Management (FARM) Program. A cornerstone of the FARM Animal Care program is the establishment of a Veterinarian-Client-Patient-Relationship where the dairy farmer consults with a veterinarian on development of treatment and recordkeeping protocols that address the proper use of antibiotics. Dairy farms will be evaluated on conformance to the standards by a certified independent expert.
National Pork Board (Iowa, U.S.)
America’s pig farmers are dedicated to raising healthy animals to help ensure a safe and healthy food supply. Pig farmers strive to reduce the need to use antibiotics by implementing production practices for infection control. They recognize the critical importance of using antibiotics responsibly in animals and humans to protect the health and well-being of both. The responsible use of antibiotics as part of an overall herd health plan is important to delivering on this commitment. America’s pig farmers will demonstrate their commitment to preventing antibiotic resistance through their support of scientific discovery and their commitment to continuous learning and improvement.
National Pork Producers Council (Iowa, U.S.)
The National Pork Producers Council (NPPC), a group of 42 affiliated state associations, will engage with U.S. delegates, as requested, to provide comments and industry information during the process of developing international standards aimed at preventing unsafe residues of veterinary drugs in food, integrating surveillance to inform risk management activities, and minimizing the development and spread of antimicrobial resistance in humans and animals. NPPC will provide industry knowledge and current scientific evidence to inform the development of these standards and utilize communications channels to increase industry knowledge and adoption of standards accepted by World Organisation for Animal Health and Codex Alimentarius.
Nebraska Department of Health and Human Services (Nebraska, U.S.)
The Nebraska Department of Health and Human Services (NDHHS) commits to expanding their antibiotic susceptibility database to identify clusters of resistant organisms by developing real-time, regional antibiograms (profiles of antimicrobial susceptibility testing results). In addition to an outbreak guidance for “nightmare bacteria” carbapenem-resistant Enterobacterales (CRE), Nebraska commits to developing a containment protocol for vancomycin-resistant Staphylococcus aureus by July 31, 2019. NDHHS will improve stewardship practices through an educational website created by ASAP (Antimicrobial Assessment and Promotion program), remote stewardship coaching, and public awareness campaigns. Visiting facilities to improve infection prevention practices, including dental clinics, will continue. The Nebraska Public Health Lab will confirm all presumed carbapenemase-producing CRE isolates detected in Nebraska laboratories.
Neem Biotech (Wales)
Neem Biotech, a pharmaceutical biotechnology company, commits to conducting research and development of a new approach to manage infections caused by pathogens on the World Health Organization Critical List. This approach targets the ways bacteria cause diseases that can result in difficult-to-treat infections and interfere with immune responses to clearing these infections. Preventing the development and spread of bacterial infections could reduce the need for antibiotics and help slow the ongoing development of resistance.
New Hampshire Department of Health and Human Services (New Hampshire, U.S.)
New Hampshire Department of Health and Human Services (DHHS) commits to continue working with Sanford Guide, providing point-of-care recommendations for infectious disease treatment, and incorporating state-specific recommendations into a mobile application for prescribers in 2020. This collaboration will allow prescribers in New Hampshire to view local antibiotic susceptibility patterns and prescribing guidelines with local data to improve antibiotic use and avoid overuse of broad-spectrum antibiotics. New Hampshire DHHS works with Sanford Guide to track antibiograms and with hospitals to track antibiotic use data. Additionally, it works with other healthcare and public health agencies to disseminate its antibiogram and clinical messaging.
New Mexico Department of Health, Epidemiology and Response Division (New Mexico, U.S.)
The New Mexico Department of Health (NMDOH) commits to engaging clinicians, hospitals, nursing homes, clinical laboratories, quality improvement organizations, and professional societies to address antibiotic resistance. NMDOH will reinforce appropriate prescribing practices with outpatient providers and provide posters and handouts educating the public about the danger of inappropriate antibiotic use. NMDOH continues its collaboration with the University of New Mexico Health Sciences Center on the extension for community healthcare outcomes (ECHO) antimicrobial stewardship project, bringing together hospital pharmacists, clinicians and infection prevention to implement CDC’s Core Elements of Antibiotic Stewardship and improve antimicrobial use by sharing best practices and antibiogram data.
New York State Department of Health (New York, U.S.)
The New York State Department of Health (NYSDOH) commits to developing a state-wide program to detect, track, and manage resistant infections at healthcare institutions. In a public-private partnership with ILÚM Health Solutions and OpGen, NYSDOH is developing a technology and genomic microbiology platform for surveillance and control of antibiotic resistance. Additionally, NYSDOH is analyzing Medicaid data to identify high prescribing areas in the state, and to improve outpatient antibiotic prescribing. Analysis of annual prescribing data over time assists NYSDOH to monitor progress in reducing antibiotic use.
Nevada Division of Public and Behavioral Health (Nevada, U.S.)
The Nevada Division of Public and Behavioral Health commits to addressing the threat of antibiotic resistance through its Healthcare-Associated Infection (HAI) Program. Program staff work with state and community partners to provide education on how to improve antibiotic use and prescribing and identify and educate those who overprescribe to decrease the inappropriate use of antibiotics in Nevada. The HAI Program created educational materials and commitment posters for healthcare providers in outpatient and acute care facility emergency rooms to share with patients on the difference between viruses and bacteria, when antibiotics are necessary, and common remedies for symptoms caused by viruses.
New Jersey Department of Health (New Jersey, U.S.)
The New Jersey Department of Health (NJDOH) Infection Control and Antimicrobial Resistance program (IC/AR) commits to providing one-on-one support to healthcare facilities to prevent, respond, and contain emerging threats such as Candida auris and to promote antibiotic stewardship. NJDOH rolled out the Antibiotic Stewardship Recognition Program (ASRP) to evaluate the impact of NJDOH’s IC/AR stewardship program. Through this program, facilities reported stewardship efforts and will be evaluated and publicly promoted based on adherence to CDC’s Core Elements of Antibiotic Stewardship. NJDOH’s Emergency Medical Services and state laboratory will also continue to monitor bacterial contamination of New Jersey ambulances to reduce spread of antimicrobial resistance threats between healthcare facilities.
Next Science (Australia)
Next Science, a company focusing on the disruption and disinfection of biofilms (microbial cells that adhere to each other on a surface), commits to the development of topical, non-antibiotic treatments that are effective against biofilms. Next Science will commercialize these products globally to allow for topical treatment of disease, which could reduce the need for antibiotic use and helps slow the development of antibiotic resistance. Additionally, Next Science is developing surface disinfectant products that will kill biofilms and prevent the spread of resistant germs in the hospital environment.
Government of Nigeria (Nigeria)
The Nigeria Centers for Disease Control (NCDC) commits to the development and scaling-up of Nigeria’s new national surveillance system for antimicrobial resistance (AMR) to inform evidence-based policy and practice for containment of AMR. NCDC also commits to the development and distribution of a national toolkit for antimicrobial stewardship in healthcare institutions, advocacy for the creation of hospital multi-disciplinary antimicrobial stewardship committees in at least 85 percent of all federal institutions, and the implementation of a national infection prevention and control program using a participatory quality development approach (PQDA) in at least 85 percent of federal healthcare institutions.
Nile’s Project (California, U.S.)
Nile’s Project commits to making healthcare safer and reducing antibiotic use in the U.S. and globally to preserve antibiotics for generations to come. As a non-profit, Nile’s Project educates the public with infection prevention awareness and tools, shares research on antibiotic resistance, and empowers the public to be antibiotics aware. Nile’s Project commits to sharing antibiotic stewardship and infection prevention solutions with healthcare professionals and healthcare facilities. Outreach emphasizes the importance of early detection and rapid identification and treatment of patients with sepsis, as well as the importance of adopting CDC’s Core Elements for Antibiotic Stewardship. Nile’s Project will continue to urge industry to deliver an affordable point-of-care rapid diagnostic tool to identify if a respiratory infection is caused by a virus or bacteria, available for healthcare and in-home use.
North American Meat Institute (Washington, D.C., U.S.)
The North American Meat Institute (NAMI) commits to employing science-based strategies by the meat and poultry industry to slow antibiotic resistance and promote antibiotic stewardship. Specifically, NAMI—which is the oldest and largest trade association representing U.S. packers and processors of beef, pork, lamb, veal and turkey—commits to improving veterinary oversight and eliminating growth uses for medically important drug compounds to protect animal and public health, while maintaining the highest standard of animal welfare practices.
Division of Public Health from North Carolina (North Carolina, U.S.)
The North Carolina Division of Public Health (NC DPH) commits to addressing the threat of antibiotic resistance by onboarding new testing that will allow for identification and characterization of two drug-resistant infections of concern: carbapenemase-producing carbapenem-resistant Enterobacterales (CP-CRE) and Candida auris. Additionally, NC DPH commits to responding to outbreaks of these organisms within 24 hours of notification and encourage improved antibiotic use through recruitment of NC hospitals into the NC Stewardship of Antimicrobial Resources (STAR) Partners initiative. These efforts will help to optimize containment of the threats and reduce the incidence of drug-resistant infections.
North Dakota Department of Health (North Dakota, U.S.)
The North Dakota Department of Health’s (NDDoH) Healthcare-Associated Infection Program commits to expanding a certificate opportunity for pharmacists to participate in a self-study training to implement, evaluate, or modify antibiotic stewardship activities at the facilities they serve in their community. This certificate program emphasizes a team approach to implement a stewardship program that will improve patient care and reduce the rates of resistance. Each pharmacist will report what aspect of antibiotic stewardship has been newly implemented because of their training. NDDoH will also continue prompt investigation of resistance threats, healthcare and patient education about antibiotic resistance, and laboratory testing to detect antibiotic resistance threats.
Northwest Antimicrobial Resistance Coalition (Washington, U.S.)
The Northwest Antimicrobial Resistance Coalition is a collaboration of the Washington Global Health Alliance, the University of Washington, Washington State University, PATH, Providence St. Joseph Health, Seattle Children’s, Harborview Medical Center, and other organizations based in the Pacific Northwest with a commitment to mitigating the effects of drug-resistant bacteria in the U.S. and around the globe. The Coalition will identify strategies that provide timely, evidence-based global antimicrobial resistance risk data to U.S. health care institutions, and effectively reduce the emergence and spread of drug-resistant germs in high-risk communities around the world. These strategies can inform the policy response to antibiotic resistance on a global scale.
Northwestern Medicine (Illinois, U.S.)
Northwestern Medicine, an integrated academic health system in the Chicago area with 4,000 physicians and 30,000 employees caring for over 1.1 million patients, is committed to improving antibiotic use to ensure that these important medications remain effective treatment options. The Antimicrobial Stewardship Quality Committee commits to using local and CDC data to reduce unnecessary antibiotic use across their system. They also commit to increased use of penicillin allergy testing to remove misdiagnoses and decrease the use of broader-spectrum antibiotics. Utilizing both of these interventions will be aimed at reducing overly broad spectrum antibiotic use and emphasize their strong commitment to better patient care and combating antibiotic resistance.
Norwegian Institute of Public Health (Norway)
The Norwegian Institute of Public Health (NIPH) commits to improving the surveillance of the key organisms responsible for driving antimicrobial resistance (AMR) in healthcare institutions, and, therefore, combating the growing risk of outbreaks of multidrug-resistant organisms. NIPH also strives to ensure that Norwegians are fully vaccinated so that illness may be avoided. NIPH commits to continue to work internationally through organizations such as the Transatlantic Taskforce on Antimicrobial Resistance and the European Union’s Joint Action on AMR and healthcare-associated infections to engage healthcare providers to improve stewardship measures as well as find common ground to implement mechanisms that can further stimulate necessary research and innovation.
NovaDigm (Massachusetts, U.S.)
Candida auris is the cause of a newly evolving fungal infection that can cause serious illness and death. In order to reduce the mortality or disability caused by by C. auris, Los Angeles Biomedical Research Institute at Harbor/UCLA (LABiomed), in collaboration with NovaDigm Therapeutics, Inc., commits to the development of a vaccine for by C. auris in order to prevent the spread of infections and save lives.
Novel Applied Pharmacy and Healthcare Services (Egypt)
The Novel Applied Pharmacy Healthcare Services (NAPHS) is a provider of pharmacy and healthcare training and consultation services in Egypt. NAPHS commits to improving antibiotic use and combating emerging resistance strains in Egyptian healthcare settings by contributing to the development of institutional antibiotic policies. It will provide four workshops every year in hospitals and facilities on how to use WHONET data to create antibiograms (antibiotic susceptibility profiles for specific bacteria). The workshops will help guide clinicians and pharmacists in selecting the best antibiotic treatment while awaiting microbiology culture and susceptibility results (test results that show an antibiotic will be effective against a germ).
Ocean Spray (Massachusetts, U.S.)
Ocean Spray’s commitment is to fund and collaborate with universities conducting research on the role of bioactive compounds found in foods, especially those high in phenolic compounds like cranberries, to assess the impact of cranberries on reducing the need for antibiotics for bacterial infections in humans and animals for the next 5 years, including research on antibiotic synergy and alternatives.
Ohio Department of Health (Ohio, U.S.)
The Ohio Department of Health (ODH) commits to addressing antimicrobial resistance (AMR) through improved detection, response, and containment of antimicrobial threats, and promotion of appropriate antibiotic use in Ohio. ODH is an active member of the Ohio Antibiotic Stewardship Advisory Committee, with other state partners including the Ohio Hospital Association, Ohio Department of Medicaid, Ohio Pharmacists Association, Ohio’s End Stage Renal Disease Network, and representatives from some of Ohio’s healthcare systems. Together, ODH will use available Behavioral Risk Factor Surveillance System and antibiotic prescribing data to develop and implement a strategic plan to reduce AMR threats in Ohio.
Ohio State University Wexner Medical Center (Ohio, U.S.)
Ohio State University Wexner Medical Center commits to developing an Antibiotic Time Out (ATO) program to promote consistent review of the appropriateness of antibiotic prescribing for its patients to combat antibiotic resistance. Clinicians will regularly review current antimicrobial therapy and consider how to modify treatment selection and duration based on culture results and clinical progress. Additionally, it is developing an electronic alert to appear in medical records 48 to 72 hours after targeted therapy initiation for provider assessment. It will examine the impact of ATO on improving patient clinical outcomes.
Oklahoma State Department of Health (Oklahoma, U.S.)
Oklahoma State Department of Health (OSDH) commits to expanding capacity to detect, prevent, and respond to antibiotic resistance in rural and urban medical facilities by educating healthcare staff through six statewide trainings on the key components of antibiotic resistance and stewardship by the end of December 2019. Antibiotic stewardship trainings will help promote awareness on the importance of de-escalation and discontinuation of antibiotic therapy, as well as education on the importance of culture surveillance data to assist with detection and response of antibiotic threats within facilities. OSDH commits to addressing the misuse of antibiotics and lack of infection prevention practices by conducting facility visits to ensure clinical staff are educated on national recommendations and have the tools to implement change at a facility level. Oklahoma is meeting CDC’s Core Elements for stewardship programs and will focus on further expansion of drug expertise and tracking components during 2019.
Omnix Medical (Israel)
Omnix Medical, a biopharmaceutical company developing novel antibiotic agents for the treatment of drug-resistant bacteria, commits to developing a novel systemic medication for the treatment of life-threatening hospital-acquired infections, to be released by 2025. Omnix technology employs fast-acting peptides, exerting a bactericidal effect, making it difficult for bacteria to develop tolerance or resistance and potentially offering a treatment where therapeutic options are scarce. Omnix is and will continue to invest and advance the research and development of novel antibiotic-agents to combat resistant bacteria.
One Drop (Canada)
One Drop, a non-profit foundation specializing in water, sanitation, and hygiene (WASH), commits to supporting WASH in healthcare facility initiatives in Haiti, Burkina Faso, Mali, and Malawi through co-financing and technical assistance. One Drop’s current financial contribution totals $8 million (U.S. dollars) across four countries, including 50 healthcare facilities, with intentions to increase this commitment. One Drop recognizes that inadequate WASH in healthcare facilities can accelerate antimicrobial resistance (AMR) due to increased risk of infections including healthcare-associated, which can lead to increased use, misuse, and overuse of antibiotics. Hence, improvements to WASH infrastructure and behaviors such as handwashing can limit AMR.
One Infinite Division, Inc. (New Mexico, U.S.)
One Infinite Division, Inc. commits to reducing infection risk by developing a sink overflow vent irrigation system that prevents the growth of bacteria in sinks to reduce microbial infections in healthcare facilities. Overflow vents catch rising water and redirect it to the drain before the sink overflows. Water that gets into the vent may not fully drain, potentially allowing bacteria to grow and pass through the air. This overflow becomes a health risk because public-use sinks, such as those in hospitals, may carry harmful bacteria. One Infinite Division’s research and development team will deliver the overflow vent irrigation system and best practice guidelines in 2020.
Opal Biosciences Ltd (Australia)
Opal Biosciences, an Australian preclinical-stage biotechnology company focused on the development of novel treatments for antibiotic-resistant or hard-to-treat infections, commits to continuing to develop urgently needed new anti-infectives for life-threatening and hard-to-treat infections. Opal’s drug, BDM-I, is a small molecule that has shown in vitro activity against many serious human pathogens including six identified by the CDC in the 2013 Antibiotic Resistance Threats Report. Opal Biosciences’ current focus is towards antibiotic-resistant Neisseria gonorrhea, where BDM-I has shown in vitro activity against all of the 2016 World Health Organization Reference strain panel, including against strains highly resistant to currently recommended antibiotics.
OpGen, Inc. (Maryland, U.S.)
OpGen, Inc., a microbial genetics analysis company, commits to providing molecular diagnostics and informatics products and services to minimize the spread and impact of antibiotic resistance. OpGen is developing a new molecular test designed to rapidly detect resistant germs and genes in less than three hours. The company is conducting clinical trials in 2019 for its direct-from-urine gene panel test and software for antibiotic resistance prediction. These products can help healthcare providers better and more rapidly treat patients with resistant infections and stop the spread of resistant germs, like carbapenem-resistant Enterobacterales.
Oregon Health Authority (Oregon, U.S.)
The Oregon Health Authority’s Healthcare-Associated Infections Program commits to addressing antibiotic resistance threats by providing technical assistance for facilities to onboard CDC’s National Healthcare Safety Network and report antibiotic use. Progress will be measured in several ways: tracking the proportion of Oregon facilities on boarded; sending annual feedback reports of outpatient oral antibiotic use rates to select Oregon health plans and analyzing the participating plans’ rates over time and against each other; and deploying educational programs for healthcare providers, veterinarians, and the public through Oregon’s Alliance Working for Antibiotic Resistance Education program (progress measured by reviewing reach, participation, and evaluations).
Government of Pakistan (Pakistan)
The Pakistan Ministry of National Health Services, Regulation and Coordination and the National Institute of Health commit to the establishment of an integrated national antimicrobial resistance (AMR) surveillance system (human, animal usage, and resistance monitoring); development of national and provincial/regional AMR reference labs in at least two provinces; reduction of the incidence of infection through effective sanitation, hygiene, and infection prevention measure through rolling out National Action Plan for AMR and supporting the provinces for development of respective Provincial Action Plans; and optimization of the use of antimicrobial medicines in human and animal health, as well as in antimicrobial sale and in the utilization audit. Pakistan also commits to complete and share the finding of Tricycle ESBL E. coli Project and the expansion of the Global Antimicrobial Resistance Surveillance System. Pakistan will also launch sentinel surveillance of invasive fungal infections such as Candida auris.
PATH (Washington, U.S.)
PATH has expertise in infection prevention and control, WASH, and laboratory capacity and surveillance in more than 70 countries around the world. PATH commits to assisting countries in responding to challenges in operationalizing a holistic antimicrobial resistance (AMR) response and supporting ministries of health in developing and operationalizing their AMR plans. Through this commitment, PATH will provide an opportunity for countries to bridge the gap between their national plans and global commitments, and promote an operational network of partners countries may turn to for support in operationalizing national AMR plans.
Pediatric Infectious Disease Society (Virginia, U.S.)
As the world’s largest organization of professionals dedicated to the treatment of infectious diseases affecting children, the Pediatric Infectious Disease Society (PIDS) commits to decreasing the use of antibiotics in pediatric settings by 20 percent by 2021. PIDS will also increase collection of pediatric-specific data by health systems and support legislative efforts to improve the research and development of new diagnostic and therapeutic environments.
Department of Health from Pennsylvania (Pennsylvania, U.S.)
The Department of Health (DOH) from Pennsylvania commits to addressing the threat of antibiotic resistance by implementing CDC’s Containment Strategy and detecting new antibiotic resistance threats through the Pennsylvania Bureau of Laboratories. Pennsylvania pledges to rapidly characterize cases of unusual resistance, conduct infection prevention and control assessments, facilitate colonization screenings, promote a coordinated response between healthcare facilities that share patients who have multi drug-resistant organisms, and recommend facility-level prevention strategies to reduce the spread of resistance. Pennsylvania will partner with labs from CDC’s Antibiotic Resistance Laboratory Network and promote the initiatives of One Health with stakeholders. Pennsylvania will continue to monitor and publish resistance trends and track the progression of state-wide infection prevention efforts through enhanced surveillance and through our support of antibiotic stewardship programs.
Perelman School of Medicine of the University of Pennsylvania and the Children’s Hospital of Philadelphia (Pennsylvania, U.S.)
The Perelman School of Medicine of the University of Pennsylvania (PENN) and the Children’s Hospital of Philadelphia (CHOP) commit to evaluate data-driven strategies that will protect their patients and serve to inform actions to combat antimicrobial resistance (AMR) in healthcare facilities nationwide. To help its providers improve antibiotic use, PENN will investigate strategies to identify excess antibiotic duration. To improve cleanliness of the hospital environment, PENN and CHOP will establish a cleaning quality assessment system, including daily inspections and unit-based performance dashboards, and conduct two studies that investigate strategies and barriers to effective cleaning. To protect patients with recurrent C. difficile infections, PENN will develop a fecal microbial transplantation program, which can help restore the gut microbiome when antibiotics are used and infections take over.
Pet Industry Joint Advisory Council (Virginia, U.S.)
The Pet Industry Joint Advisory Council (PIJAC) and the broader pet care community commit to the development and adoption of Recommendations for Judicious Use of Antimicrobials in Companion Animal Care. These recommendations will establish best management practices for antibiotic use that will encourage responsible stewardship while protecting human and animal health and well-being. PIJAC and its partners will provide outreach and education to the pet care community and the general public on these best practices to encourage implementation.
Petco (California, U.S.)
Petco will continue a focus on judicious use of antibiotics by not allowing prophylactic use of antibiotics in our supply chain and supporting veterinary oversight for access to antibiotics in its 1,500 locations across the U.S. and online channels.
Pew Charitable Trusts (Pennsylvania, U.S.)
The Pew Charitable Trusts uses evidence-based, non-partisan analyses to solve today’s challenges. To combat antibiotic resistance, Pew will: strive to reduce inappropriate antibiotic use across healthcare settings by supporting efforts to track prescribing and establish data-driven targets for improving inpatient antibiotic use; coauthor research to support outpatient stewardship; promote judicious antibiotic use across the animal agriculture industry by developing sustainable stewardship standards and publishing research on the impact of different management and disease prevention practices on the need for antibiotics; and spur new antibiotic discovery by providing the publicly-available Shared Platform for Antibiotic Research and Knowledge (SPARK) to enable scientists around the world to collaborate on research targeting the hardest-to-treat bacteria.
Pfizer Inc. (New York, U.S.)
Pfizer Inc. commits to advancing its innovative surveillance programs, and ensuring that data from ATLAS—one of the largest, most accessible AMR surveillance programs in the world— and the SENTRY fungal surveillance program, are publicly regularly updated for public availability. ATLAS monitors real-time changes in pathogen resistance and detects trends in multi-drug resistance longitudinally. Pfizer will also work to expand access to its diverse portfolio of medicines and vaccines to treat and help prevent resistant infections worldwide, with a focus on those pathogens most difficult to treat with current antibiotics.
PharmAccess Group (Netherlands)
SafeCare is an international initiative of PharmAccess Group and constitutes a practical methodology that tracks, acknowledges, and certifies incremental clinical quality improvements in health facilities in resource restricted settings. SafeCare commits to continuing to provide a digital quality improvement platform for healthcare providers to improve their infection prevention standards and policies to reduce antimicrobial resistance through benchmarking, sharing of best practice examples, and real-time progress information. The platform will be accessible upon request to stakeholders so that donors can follow the progress of facilities they support and governments can access the data to make informed decisions and for resource allocation.
PharMerica (Kentucky, U.S.)
PharMerica, a post-acute and long-term care full-service pharmacy, commits to partnering with the CDC in distribution and analysis of pharmacy dispensing data from December 2016 to January 2018. Through this project, skilled nursing facilities will be able to track antibiotic use, facilitate antibiotic reporting in nursing homes, compare rates to national standards, and identify opportunities to improve antibiotic prescribing in nursing homes.
Phibro Animal Health (New Jersey, U.S.)
Phibro Animal Health commits to developing innovative nutritional products and vaccines for diseases affecting poultry, swine, and cattle to build herd and flock immunity and provide unique formulations that do not require refrigeration. Phibro will continue to communicate with consumers regarding animal antibiotic use, vaccines and animal health, advocating for a one health approach to antibiotic stewardship. Phibro will provide information to consumers through the Explore Animal Health website and on social media.
Pison Stream Solutions (New York, U.S.)
Pison Stream Solutions—a company that develops coatings and green technology—commits to continuing to pilot solutions for antimicrobial coating technology advancements. Pison commits to continued research and development of antimicrobial powder coatings with high efficacy ratings and bacterial reduction available in 2020. Pison will collaborate with product manufacturers to communicate antimicrobial resistance results to the public from its research and development and Pison’s mission to prevent bacterial growth on highly-contaminated surfaces.
PixCell Medical Technologies Ltd. (Israel)
PixCell Medical Technologies Ltd., a developer of portable medical diagnostic products for point-of-care testing, commits to improving appropriate antibiotic use by providing real-time, point-of-care diagnostics. PixCell will invest in the development and commercialization of its products, including assays for differentiating between bacterial and viral infections, so that healthcare professionals can have access to diagnostic information before prescribing antibiotics. Additional assays will be developed to further advance PixCell’s mission of improving clinical outcomes by providing blood test results when and where they are needed.
Premier, Inc. (Ohio, U.S.)
Premier, a healthcare improvement company with a network of approximately 4,000 U.S. hospitals and health systems and approximately 165,000 other providers and organizations, is committed to continue to reduce preventable harms caused by hospital-acquired infections and inappropriate antimicrobial use. Premier will work with more than 500 members in its Hospital Improvement Innovation Network (HIIN), funded by Centers for Medicare & Medicaid Services, to provide education, technical assistance, and evidence-based strategies to implement all seven of the CDC’s Core Elements of Hospital Antibiotic Stewardship Programs and reduce the rate of healthcare-associated C. difficile by 20 percent by March 2019. Premier will continue to work alongside healthcare providers to co-develop, scale, and enhance its data-driven solutions, research and educational services, and best practices to improve antimicrobial stewardship across the U.S. In doing so, Premier will conduct follow-up research to measure hospital progress in improving antimicrobial use and publish findings in 2019. Premier also commits to enabling increased reporting of antibiotic use data by Premier members through its data platform; using deep analytics with peer-to-peer benchmarks to understand usage patterns; and applying its collaborative methodology to target, implement and measure improvement efforts.
ProAgni (Australia)
ProAgni, a manufacturer of antibiotic-free animal food products and systems for cattle and sheep, commits to reducing antibiotic use in animal production in Australia, New Zealand, and the United States. ProAgni has developed content on antibiotic resistance and the responsible use of antibiotics in animal production. It will present approximately six presentations at industry and academic events within the next 12 months. ProAgni is collaborating with Australian and American education and scientific institutions on research and development projects to further understand antibiotic resistance and agriculture. Additionally, ProAgni has developed antibiotic-free feed supplements to improve digestion and support healthy animals.
PointClickCare (Canada)
PointClickCare is committed to helping understand antibiotic prescribing practices in long term care facilities. We analyzed millions of antibiotic orders and admission records in thousands of nursing facilities across the United States. We will continue to mine the data and share insights with CDC to support efforts to improve care.
QIAGEN (Netherlands)
QIAGEN’s core mission is making improvements in life possible that will last. In the fight against the global spread of antibiotic-resistant (AR) pathogens, QIAGEN commits to developing products aimed at improving antibiotic resistance surveillance and research. This includes negotiating the release of private AR sequence databases, integration with public databases, and creating new curated genomics resources on AR for the research and public health community. QIAGEN will also develop new targeted assays and workflows for AR, and will launch an annual Early-Career Research Innovation Award designed to provide training, consulting, software and reagent support to researchers focused on AR.
RB Health (US) LLC (New Jersey, U.S.)
RB Health, LLC—a health and hygiene company operating in more than 60 countries—commits to supporting appropriate antibiotic use by reaching healthcare professionals in high-prescribing areas in the U.S., focusing on urgent care clinics. RB will support health care practitioners in communicating to patients that the appropriate treatment for viral coughs and colds is not antibiotics. RB will distribute patient educational material on the appropriate use of antibiotics to urgent care centers and primary care offices across the U.S. RB will work with the Urgent Care Association on antibiotic stewardship and the reduction of inappropriate antibiotic prescriptions.
Republic of Korea Ministry of Health and Welfare (Korea)
The Republic of Korea Ministry of Health and Welfare commits to implementing a standardized surveillance system in the country that complies with World Health Organization’s Global Antimicrobial Resistance Surveillance System. The Ministry will implement a multi-sectoral joint project against antimicrobial resistance (AMR) that will include collaboration with six different ministries to research AMR and provide strategies for action.
Rhode Island Department of Health (Rhode Island, U.S.)
The Rhode Island Department of Health (RIDOH) commits to educating the public and providers in acute care, post-acute care, and ambulatory care settings on appropriate antibiotic use by fostering dialogue, education, and awareness. RIDOH’s Antimicrobial Stewardship and Environmental Cleaning Task Force is addressing the misuse of prescription medications, including antibiotics, by working in partnership with local community pharmacies to promote an Antibiotic Take Back Campaign to be kicked off in conjunction with the Drug Enforcement Agency’s National Drug Take Back Day in October 2019. This follows a successful 2018 strategy of encouraging consumers to properly dispose of antibiotics at state and local police locations statewide.
Roche Diagnostics (New Jersey, U.S.)
One of the world’s largest diagnostics companies, Roche Diagnostics, commits to implementing antibiotic use education and awareness projects among over 4,000 employees as well as healthcare providers and clinical partners. Roche will also invest in diagnostic solutions and assessment tools that identify or rule out resistant pathogens, and measure the effectiveness of an antibiotic therapy.
Rush University Medical Center (Illinois, U.S.)
Rush University Medical Center, with over 47,000 annual patient admissions, is committed to reducing hospital-onset C. difficile infections by 25 percent by December 2019. C. difficile infections can cause deadly diarrhea and are associated with antibiotic use, which drives antibiotic resistance. Rush will use a multimodal approach to drive down rates, including infection prevention, antibiotic use programs, environmental services, hospital quality and safety, clinical informatics, and microbiology labs.
Sackler Institute for Nutrition Science (New York, U.S.)
The Sackler Institute for Nutrition Science commits to support the gathering and sharing of knowledge on the use of antibiotics in animal food production. They will compile and publish scientific advances on antimicrobial resistance (AMR) in animal food industry to clarify the relationship between AMR in animal food production, the various pathways of pathogen transmission to humans, alternatives to antibiotics in animal food industry, and ways of communicating information to consumers.
Save the Children (Connecticut, U.S.)
Save the Children commits to expanding its safe drinking water, sanitation, and hygiene (WASH) strategy in healthcare facilities investments to at least five new countries and advocating globally and nationally, including convening several national partners attending World Health Organization (WHO) meetings, as part of its global, three-year WASH strategy (2019-2021). Save the Children will document the cost of implementation to share with the public and leverage its partnerships to advocate for more investments, research, and activities for WASH in healthcare facilities.
SCYNEXIS, Inc. (New Jersey, U.S.)
Invasive fungal infections are often deadly and typically target patients with compromised immune systems. SCYNEXIS is developing a novel IV/oral antifungal medication for the treatment of Invasive Aspergillosis, Invasive Candidiasis, and Vulvovaginal Candidiasis, including patients with resistant pathogens. SCYNEXIS is committing to initiate studies of this antifungal in the treatment of patients with Invasive Aspergillosis and patients with fungal infections that are resistant to current therapies, including patients with Candida auris.
Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS) Collaborative (Missouri, U.S.)
Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS) Collaborative—a collaborative of more than 50 U.S. children’s hospitals focused on antimicrobial use best practices —commits to reducing inappropriate antibiotic use in its hospitalized children by 20% by December 2021 as measured through quarterly point prevalence surveys. SHARPS will increase the number of SHARPS Collaborative hospitals conducting antimicrobial stewardship at time of discharge by 50% and the number of its hospitals submitting pediatric specific data by 50%. Additionally, SHARPS will ensure stewardship activities are implemented in 50% of its hospitals caring for hospitalized children by the end of 2021.
SNV (The Netherlands)
SNV, an international non-profit organization that strives to raise incomes and improve basic service for people living in poverty, commits to integrating safe drinking water, sanitation, and hygiene (WASH) in healthcare facilities into relevant activities and operations, advocating globally and nationally, and developing its internal WASH in healthcare facility approach.
Society for Healthcare Epidemiology of America (Virginia, U.S.)
The Society for Healthcare Epidemiology of America (SHEA) commits to improving antibiotic use through expanded educational efforts. SHEA will increase content for healthcare professionals by 25 percent, increase scholarship funds for antibiotic stewarship training by 100 percent, develop antibiotic use guidance for long-term care settings, publish a white paper on antibiotic stewardship research priorities, and launch an education campaign for new Congressional members.
Society for Pediatric Urgent Care (Virginia, U.S.)
The Society for Pediatric Urgent Care (SPUC) commits to decreasing inappropriate antibiotic use in pediatric urgent care organizations by 20% by December 2019. SPUC will work with CDC and the Antibiotic Resistance Action Center (ARAC) to continue an ongoing project that examines and improves the use of antibiotics in pediatric urgent care by implementing quality improvement initiatives. The project will collect, analyze, and feedback data monthly to participating providers and centers through 2019 to guide each organization’s quality improvement efforts.
Society of Infectious Disease Pharmacists (Illinois, U.S.)
The Society of Infectious Disease Pharmacists (SIDP) will promote the appropriate use of antibiotics among its more than 1500 members by expanding global stewardship provider education in acute and long-term care, providing antibiotic stewardship certificate programs, securing commitments from U.S. acute care hospitals to adopt the stewardship practices outlined in pharmacy action posters, a collaboration between CDC, SIDP, and the American Society of Health-System Pharmacists, and sponsoring an Antibiotic Take Back Day campaign in partnership with community pharmacists.
Department of Health and Environmental Control from South Carolina (South Carolina, U.S.)
The Department of Health and Environmental Control from South Carolina commits to addressing the threat of antibiotic resistance by requiring hospitals and laboratories to report multi-drug resistant organisms; testing for drug sensitivities for carbapenem-resistant Enterobacterales, carbapenem-resistant Pseudomonas aeruginosa, Candida auris, vancomycin-intermediate/resistant Staphylococcus Aureus (VISA/VRSA), and drug susceptibility test genotyping for resistant tuberculosis through our state laboratory; conducting outreach to sentinel hospital labs highlighting CDC’s Antibiotic Resistance Laboratory Network testing in 2019; and planning annual regional and statewide meetings to improve antibiotic stewardship in 2019.
South Dakota Department of Health (South Dakota, U.S.)
South Dakota Department of Health (SDDOH) commits to continuing to implement CDC’s Infection Control Assessment and Response (ICAR) assessments and Targeted Assessment for Prevention (TAP) reports in outbreak-affected facilities. SDDOH will provide technical assistance to healthcare providers, enhance collaborative partnerships, strengthen analysis, and enhance infection control in outbreak-affected facilities. SDDOH will analyze and share CDC’s National Healthcare Safety Network (NHSN) data quarterly with NHSN’s HAI group to guide prevention activities, outbreak response, and identify priorities to reduce healthcare-associated infection and antibiotic-resistant pathogen transmission in South Dakota. SDDOH will collaborate with the South Dakota Public Health Laboratory to detect multidrug-resistant germs, measure prevalence, and provide guidance to direct infection prevention activities in facilities across the state.
SpeeDx (Australia)
SpeeDx, a diagnostic manufacturer, commits to developing novel diagnostic tools that go beyond detection, providing therapeutic guidance to clinicians for improved patient care and antimicrobial stewardship. SpeeDx will promote the use of resistance-guided therapy to customize treatments and ensure infections are susceptible to the antibiotic prior to treatment. SpeeDx is collaborating with researchers, clinicians, organizations and industry partners as part of a Transformational Research Hub, funded by the Australian government, to address the increase of antimicrobial resistance.
Summit Therapeutics (United Kingdom)
Summit Therapeutics is committed to discovering and developing new antibiotics for serious infectious diseases. Once easy to cure infections are becoming deadly due to increasing antibiotic resistance and a lack of truly new antibiotics coming to market. Summit believes by developing new antibiotics for specific infections, misuse of antibiotics could be reduced and resistance kept at bay. The Company is currently developing antibiotics for C. difficile, gonorrhea and ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species).
TB Alliance (New York, U.S.)
TB Alliance—a non-profit organization dedicated to the discovery, development, and delivery of better, faster-acting, and affordable tuberculosis (TB) drugs—commits to developing new drug regimens to treat TB, including drug-resistant TB. TB continues to be the largest driver of deaths caused by antibiotic resistance globally. TB Alliance will partner with governments, academic institutions, the private sector, and philanthropic organizations to target registration and introduction in at least 30 countries where extensively drug resistant (XDR)-TB is high risk. Progress will be measured by the global use of regimens containing pretomanid, an FDA-approved TB drug used for treatment of XDR-TB, in combination with bedaquiline and linezolid.
Temple University Hospital Antimicrobial Stewardship Program (Pennsylvania, U.S.)
Temple University Hospital (TUH) Antimicrobial Stewardship Program (ASP) is committed to improve patient safety and health outcomes by promoting appropriate antibiotic use through focused stewardship interventions and will continue to enhance its partnership with front-line prescribers. By June 2019, TUH ASP commits to focusing on optimizing antibiotic prescribing in patients with a reported penicillin allergy through development of a TUH evidence-based guideline for evaluating reported penicillin allergy and via prospective review and feedback of aztreonam, a broad-spectrum antibiotic, and continuing efforts to reduce medication errors through TUH ASP’s anti-retroviral stewardship program.
Tennessee Department of Health (Tennessee, U.S.)
The Tennessee Department of Health (TDH) commits to improving antibiotic use with a required state mandate that all acute care facilities report antibiotic use into CDC’s National Healthcare Safety Network Antimicrobial Use and Resistance Module, beginning with the largest facilities by January 1, 2021. TDH will use the data to identify and reach out to high prescribers statewide for targeted antibiotic stewardship interventions. TDH also commits to performing statewide targeted infection control assessments (TICAs) at all types of healthcare facilities. TICAs include in-person visits from TDH infection preventionists to provide consultation on how to improve their practices.
Texas Department of State Health Services (Texas, U.S.)
The Texas Department of State Health Services (DSHS) commits to increasing molecular testing for antibiotic resistance mechanisms as part of its role as a regional lab within CDC’s Antibiotic Resistance Laboratory Network. DSHS will also increase activities to prevent spread of antibiotic resistance by educating public health partners and healthcare providers, and promoting collaborative efforts between them, to enhance stewardship practices in acute care, long-term care, and outpatient healthcare settings.
TGen North (Arizona, U.S.)
TGen North commits to developing a region-wide antibiotic resistance phenotype and genotype knowledgebase over the next three years. The AMR knowledgebase is essentially an interactive and continually updated relational database capturing both phenotypic and genetic information on presence and incidence of AMR for healthcare and public health tracking in the Southwest U.S. This knowledgebase will allow for improved tracking of resistance genes within the region, enabling the identification of resistance trends and guiding the development of genomic-based diagnostic tools for clinical medicine and public health laboratories.
Thermo Fisher Scientific (Massachusetts, U.S.)
Thermo Fisher Scientific commits to developing nearly 100 products and devices for the rapid diagnosis of infections, optimization of antibiotic treatment, and monitoring of resistance patterns by the end of 2019. Thermo Fisher is also partnering with global antimicrobial susceptibility surveillance programs to provide a standardized tool to support public health and national reference laboratories to actively monitor the increasing global threat of antimicrobial resistance. By partnering with pharmaceutical companies in new drug development, Thermo Fisher will continue to deliver innovative and targeted products and devices required for hospitals to implement new antibiotics and save lives.
Tiny Earth (Wisconsin, U.S.)
Tiny Earth, a network of instructors and students from more than 250 high schools and colleges across 45 U.S. states and in 15 countries focused on crowdsourcing antibiotic discovery from soil, commits to creating public service announcements to raise awareness about antimicrobial resistance and stewardship, illuminating simple actions people can take to help turn the tide. Tiny Earth will also leverage the discovery potential of its global network of student-scientists to continue the hunt for novel antibiotics.
Trans-Atlantic Taskforce on Antimicrobial Resistance (Georgia, U.S.)
Through cooperation and collaboration between the USA, Canada, Norway and the European Union, the Trans-Atlantic Taskforce on Antimicrobial Resistance (TATFAR) is committed to showing leadership to reduce the threat from antimicrobial resistance (AMR) by working together in three key areas: 1) Improving appropriate therapeutic use of antimicrobial drugs in medical and veterinary communities; 2) Preventing healthcare- and community-associated drug-resistant infections; 3) Developing strategies for improving the pipeline of new antimicrobial agents. TATFAR endorses the AMR Challenge and will continue to work to raise awareness and support action by its partners and networks through initiatives such as the AMR Challenge to garner additional commitments and action that further progress the fight against AMR.
Trinity Health (Michigan, U.S.)
Trinity Health, a network that includes 94 hospitals and 120 continuing care locations across 22 states, commits to reducing incidences of healthcare-associated infections (HAIs) by implementing best-in-class infection prevention-and-control protocols; ensuring the appropriate use of antibiotics through stewardship at the local level guided by oversight and support from system-level leaders; implementing an improved standard environmental services program model proven to help avoid HAIs; and by developing clinical decision support processes and tools to optimize the use and stewardship of laboratory diagnostics, therapeutics, and vaccines.
Try This First, Inc. (California, U.S.)
Try This First, Inc., the developer of an over-the-counter treatment for children experiencing earaches, commits to investing more than $275,000 toward data sharing and awareness campaigns about the limited effectiveness of treating ear pain with oral antibiotics. The company will share with pediatricians, pharmacists, pediatric nurse practitioners, and school nurses data provided by the Centers for Disease Control and Prevention and the National Institutes of Health on the excessive use of antibiotics to combat ear infections. The company will also share the Watchful Waiting protocol, recommended by the American Academy of Pediatrics, to help resolve child ear pain within 72 hours on its own.
Typhoid Vaccine Acceleration Consortium (Maryland, U.S.)
The Typhoid Vaccine Acceleration Consortium (TyVAC) commits to furthering the data available regarding typhoid conjugate vaccines (TCV), elevating typhoid and TCVs as urgent public health priorities, and supporting countries that want to introduce TCV, particularly as a means to help prevent and control outbreaks of drug-resistant typhoid. TyVAC is working in Bangladesh, Burkina Faso, Nepal, Malawi, Pakistan, and Zimbabwe, with the possibility of adding new countries during the project.
Uganda Catholic Medical Bureau (Uganda)
Uganda Catholic Medical Bureau (UCMB), the health office of the Roman Catholic Church in Uganda, commits to continuing to support safe drinking water, sanitation, and hygiene (WASH) in healthcare facilities by assessing, supervising, and training all levels of staff in its 265 health centers and seven hospitals. UCMB commits to continue with technical supervision of 297 health facilities with emphasis on provision for people with mobility disability, hand hygiene, and safe segregation of waste, and advocate and lobby for WASH using existing church and coordination structures.
United States Agency for International Development (Washington, D.C., U.S.)
United States Agency for International Development (USAID) commits to strengthening health systems and tracking safe drinking water, sanitation, and hygiene (WASH) improvements in healthcare facilities. USAID will support WASH in health facilities within national and local contexts to achieve objectives embedded in the U.S. Global Health Security Strategy and the U.S. Global Water Strategy, as well as USAID’s Policy Framework. Beginning in 2020, USAID will track a suite of indicators measuring WASH in health systems in U.S. Government-supported sites to support evidence-based investment decisions, achieve objectives related to preventing antibiotic resistance, and increase the quality of integrated healthcare services.
U.S. Agency for Healthcare Research and Quality (Washington, D.C., U.S.)
The U.S. Agency for Healthcare Research and Quality (AHRQ) supports research and implementation projects to develop improved methods for combating antibiotic resistance (AR) and accelerate adoption of evidence-based practices in three core domains of the fight against AR: promoting antibiotic stewardship, preventing transmission of resistant bacteria, and preventing healthcare-associated infections (HAIs) in the first place. The AHRQ Safety Program for Improving Antibiotic Use has made significant progress in adapting AHRQ’s Comprehensive Unit-based Safety Program (CUSP), which has been highly effective in preventing HAIs, to improve antibiotic use and promote antibiotic stewardship in multiple health care settings: acute care hospitals, long-term care facilities, and ambulatory care settings.
U.S. Biomedical Advanced Research and Development Authority (Washington, D.C., U.S.)
In a global partnership with the National Institutes of Health, the Gates Foundation, the Wellcome Trust, and the United Kingdom Government, the U.S. Biomedical Advanced Research and Development Authority (BARDA) continues to support the Combatting Antibiotic Resistant Bacterial Accelerator (CARB-X) through a cooperative agreement with Boston University to accelerate global antibacterial innovation. Including CARB-X, BARDA supports the end-to-end research, development and regulatory approval of 45 promising antibacterial products from lead optimization through product approval. To date, CARB-X has provided $87 million to support 34 innovative early stage projects, including 10 new classes of antibiotics, 10 non-traditional therapies, and six rapid diagnostics, while BARDA has provided over $1 billion to support the clinical development of 12 advanced stage antibacterial products, two of which, Vabomere and Zemdri, were approved for use by the FDA.
U.S. Centers for Disease Control and Prevention (Georgia, U.S.)
The U.S. Centers for Disease Control and Prevention (CDC) is a global leader in the fight against antibiotic resistance. CDC is leading The AMR Challenge with the U.S. Department of Health and Human Services (HHS) to build on its domestic and global commitments to combat antibiotic resistance, and to engage stakeholders from around the world to participate in the fight. Only through our collective efforts will we be able to make a difference against this growing problem that has the potential to impact all of us. In order to protect patients and communities from resistance, CDC will continue to leverage its unique expertise to: collaborate with other countries to prevent and contain the global spread of resistance threats; partner with states and countries to improve antibiotic use; enhance detection and tracking in the U.S., including scaling up cuttingedge laboratory technology through CDC’s Antibiotic Resistance Laboratory Network; pilot new and innovative approaches to combat resistance domestically and globally; and share isolates via the CDC and FDA Antibiotic Resistance Isolate Bank to inform the development of new drugs and diagnostics.
U.S. Centers for Medicare and Medicaid Services (Washington, D.C., U.S.)
The U.S. Centers for Medicare and Medicaid Services (CMS) Hospital Improvement Innovation Networks (HIIN) and Quality Innovation Network/Quality Improvement Organizations (QIN/QIOs) works with recruited hospitals to prevent device and procedure-associated infections and C. difficile in hospitals. Quality Innovation Networks-Quality Improvement Organization (QIN-QIOs) work with recruited nursing homes to improve upon several areas of patient safety including tracking, reporting, and preventing C. difficile infections in this setting. Additionally, the CMS Merit-Based Incentive Payment System (MIPS) supports CDC’s development of an antibiotic stewardship training course for healthcare professionals. Further, CMS completed and released new Interpretive Guidance in 2017 which includes how to survey for antibiotic stewardship in nursing homes.
U.S. Department of Agriculture (Washington, D.C., U.S.)
U.S. Department of Agriculture Animal and Plant Health Inspection Service (APHIS) launched an ongoing antibiotic resistance (AR) pilot project in 2018 with the goals of monitoring AR profiles in animal pathogens from veterinary diagnostic laboratories across the U.S. using standard test methods, and developing standardized antimicrobial susceptibility data transmission and sharing processes. USDA APHIS also created two modules—Antibiotics in Animals and Veterinary Feed Directive—through the National Veterinary Accreditation Program (NVAP) that play key roles in USDA’s AR global education and outreach efforts. For over two decades, USDA APHIS’ National Animal Health Monitoring System (NAHMS) has collected data on antimicrobial use and resistance in periodic national studies of multiple food animal species. In 2017, NAHMS conducted two targeted studies of antimicrobial use and stewardship practices on swine operations and cattle feedlots. USDA’s Food Safety and Inspection Service (FSIS) continued to expand the animal species and commodities to test for regulated pathogens. All the pathogens including Salmonella, Campylobacter, and Shiga toxin-producing E. coli (STEC) that originate from regulatory samples are subject to whole genome sequencing (WGS) and further characterization including AR. FSIS continues to administer the National Antimicrobial Monitoring System (NARMS) cecal sampling program and subjects all Salmonella and Campylobacter and a subset of E. coli and Enterococcus isolates to WGS. In addition, all the FSIS isolates continue to be phenotypically tested for antimicrobial susceptibility. In addition, all the FSIS isolates continue to be phenotypically tested for antimicrobial susceptibility.
U.S. Department of Defense (Washington, D.C., U.S.)
The Multidrug-resistant organism Repository and Surveillance Network (MRSN) is the U.S. Department of Defense’s (DoD) reference laboratory for multidrug-resistant pathogen characterization throughout the military healthcare system. In addition, it works collaboratively with the DoD Infectious Disease Research Program (IDCRP) on wound-specific characterization and the Veterans Health Administration (VA) on pathogen information data sharing as well as with the Drug Development arm of Experimental Therapeutics and Bacterial Disease Branches at the Walter Reed Army Institute of Research (WRAIR) for therapeutics development. As part of the Antibiotic Resistance Monitoring and Research (ARMoR) program, inclusive of the MRSN, Navy Marine Corps Public Health Center-Epi Data Center (NMCPHC-EDC), and Army Pharmacovigilance Center (PVC), the program compiles and analyzes both pathogen-specific trends, transmission patterns, and the associated use of antibiotics as part of the DoD-wide antimicrobial stewardship program. It is through this program that DoD was able to implement centralized pathogen and antibiotic use centralized reporting to NHSN AR and AU modules. Furthermore, the DoD works with CDC as an AR Lab Network regional hub.
U.S. Department of Health and Human Services Office of Global Affairs (Washington, D.C., U.S.)
The U.S. Department of Health and Human Services (HHS) Office of Global Affairs (OGA) facilitated bilateral meetings where antimicrobial resistance (AMR) was a priority topic of discussion between HHS and the Ministries of Health from the European Union, Netherlands, Japan, Canada, and Argentina. Additionally, in July 2017 OGA hosted leaders from the World Health Organization (WHO) for a discussion on policy and efforts with the Combating Antibiotic Resistant Bacteria (CARB) Task Force members. A second visit with WHO principles was hosted in March 2018 to introduce new WHO leadership on AMR to HHS and U.S. Department of Agriculture principles.
U.S. Department of State (Washington, D.C., U.S.)
The Department of State has successfully advanced a multi-year comprehensive, strategic, and innovative approach to enable immediate and lasting action globally to combat antimicrobial resistance (AMR). In addition to multilateral efforts culminating in high-level political support globally for multi-sectoral action on AMR, the Department of State has pursued pioneering efforts to enhance individual, community, and country action. The Department of State co-hosted the first forum for faith-based organizations to self-identify their roles and responsibilities in addressing the AMR challenge, titled “Combating the Emergence and Spread of Antimicrobial Resistance: A Workshop to Strengthen Faith-Based Engagement.” In addition, the Department of State also promoted policy support (including at United Nations Environment Assembly 3) and technical collaborations to advance a scientific agenda that will enable all communities—including in low-resource settings—to understand and implement evidence-based interventions related to the environmental component of AMR. As a 2018-2019 Science Envoy, Dr. Michael Osterholm, Ph.D., MPH will work to support key priorities including addressing the emergence and spread of drug-resistant disease globally.
U.S. Department of Veterans Affairs (Washington, D.C., U.S.)
The U.S. Department of Veterans Affairs (VA) has collaborated with CDC to fund the CDC/VA Infection Control Research Network to evaluate high priority healthcare-associated infections/antibiotic resistance prevention focus areas in 15 VA medical centers and their affiliated skilled nursing facilities. The VA was funded by CDC to design and pilot test a registry that would allow for identification of patients with previous multidrug-resistant organisms (MDRO) to be identified at admission to any VA facility allowing for a more complete and timely institution of MDRO infection control precautions at hospital admission. The VA is also working with CDC to scale-up stewardship interventions in VA outpatient and acute care settings, and to refine methods for measuring hospital antibiotic use in the National Healthcare Safety Network. And lastly, the VA, in collaboration with CDC and the University of Utah, supported a hospital-level analysis on acute-care methicillin-resistant Staphylococcus aureus (MRSA) admission prevalence, and found that decreased MRSA acquisition rates lead to decreased importation and further abates acquisition.
U.S. Food and Drug Administration (Washington, D.C., U.S.)
The U.S. Food and Drug Administration (FDA) has issued several guidance documentsexternal icon on recommended clinical trial designs for evaluating antibacterial drugs. FDA has been working to implement the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) pathway created under the 21st Century Cures Act and recently published a draft guidance, “Limited Population Pathway for Antibacterial and Antifungal Drugs Guidance for Industry,” which, when finalized, will describe an approval pathway for certain antibacterial drugs to treat serious or life-threatening infections in limited populations of patients with unmet needs. FDA continues collaboration with CDC on the Antibiotic Resistance Isolate Bank. FDA also launched the susceptibility test interpretive criteria (“breakpoints”) webpagesexternal icon required by the Cures Act, which provided FDA with the tools to modernize and streamline updating of breakpoints information for antimicrobial drugs. FDA is also implementing the Generating Antimicrobial Incentives Now (GAIN) Act provisions of the Food and Drug Administration Safety and Innovation Act (FDASIA) to help facilitate antibacterial drug development. In this connection, it has granted 163 Qualified Infectious Disease Product (QIDP) designations, including approximately 80 designations for novel drugs, and has approved 14 drug products with QIDP designation. Lastly, FDA supports efforts to monitor antimicrobial drug use in food-producing animals through collection of nationally representative on-farm data on antimicrobial use practices and resistance. To build on the progress already made, FDA recently published a blueprint on the additional steps it will take to address antimicrobial resistance in veterinary settings. Serving as FDA’s new five-year plan, this blueprint iincludes Center for Veterinary Medicine’s key goals, objectives and actions for fiscal years 2019 – 2023.
U.S. National Institutes of Health (Washington, D.C., U.S.)
In 2014, the U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) issued its report “NIAID’s Antibacterial Resistance Program: Current Status and Future Directions” to describe the Institute’s research portfolio and outline a combination of innovative approaches based on the latest scientific advances. NIH/NIAID is actively pursuing research in these innovative areas, including bacteriophage therapy, microbiome-based therapeutics, and immune-based strategies to be pursued. In 2016, NIH/NIAID published a paper in the Journal of the American Medical Association reinforcing the need for novel approaches to combat antibiotic resistance. The NIH/NIAID-supported Antibacterial Resistance Leadership Group (ARLG) is pioneering a robust clinical research agenda on antibacterial resistance, including novel drugs, treatment regimens, and diagnostics. With NIH/NIAID support, scientists are developing and testing new tools to rapidly detect bacteria, and determine their sensitivity and/or resistance to antibiotics, and distinguish bacterial and viral infections at the patient point-of-care.
United Nations Foundation (Washington, D.C., U.S.)
The United Nations Foundation commits to leveraging its convening power, advocacy and communications expertise, access to UN agencies, relationships with the private sector, civil society organizations, and other key stakeholder groups, to secure political will and financial support to combat antimicrobial resistance (AMR). UN Foundation will continue to convene AMR community calls to coordinate advocacy and policy efforts on a monthly basis to provide a platform for all stakeholders to share lessons learned and rally around priority initiatives, and work with partners to organize and host events to elevate the AMR agenda amongst relevant stakeholders, including the November 2018 Call to Action on AMR in Ghana. They will further the work with the private sector and policy community to mobilize resources to build a sustained, neutral and broad based multi-stakeholder platform for engagement that cuts across environment, animal, and human health, and across private, public and civil society sectors.
United States Pharmacopeial Convention (USP) (Maryland, U.S.)
United States Pharmacopeial Convention (USP) commits to develop and revise science-based quality standards for antibiotics. USP will support inclusion of medicine quality assurance in AMR national action plans and build coalitions for action, through the Medicines We Can Trust campaign. USP will work within the USP-APEC Center of Excellence to train regulators and industry on quality assurance practices that help combat AMR. USP will generate evidence on the link between poor quality medicines (which do not meet regulatory requirements for quality) and antibiotic resistance. Through the USAID-funded, USP-implemented Promoting the Quality of Medicines program, USP will continue working with regulators and drug manufacturers to expand access to quality-assured essential antibiotics; strengthen quality systems to prevent, detect, and respond to poor quality antibiotics; and contribute to surveillance efforts tracking antibiotic quality.
United States Public Interest Research Group (Colorado, U.S.)
The United States Public Interest Research Group (U.S. PIRG) is a federation of U.S. non-profit organizations that advocate on behalf of public interest on issues such as public health, product safety, and consumer protection. U.S. PIRG commits to continue mobilizing consumers and healthcare providers to encourage major food companies to source only meat raised without the routine use of antibiotics. U.S. PIRG is working with the Antibiotics Off the Menu coalition to convince the top six restaurants in the U.S. to make such commitments by 2025. Over the years, U.S. PIRG and its state affiliates have worked to reduce antibiotic use in food animal production.
University of Illinois at Chicago and University of Illinois Hospital and Health Sciences System
(Illinois, U.S.)
As a CDC Prevention Epicenter, the University of Illinois at Chicago and University of Illinois Hospital and Health Sciences System (UIH) is committed to prevention of infections and decreasing inappropriate antimicrobial use. UIH will continue to improve antibiotic use for specific illnesses, like upper respiratory infections, among its over 600 providers; reduce sepsis-related deaths; and participate in clinical trials of new antibiotics.
University Illinois at Chicago (Illinois, U.S.), University of Iowa (Iowa, U.S.), University of Maryland (Maryland, U.S.), Emory University (Georgia, U.S.), University of Utah (Utah, U.S.)
CDC’s Prevention Epicenters at the University of Illinois at Chicago, University of Iowa, University of Maryland, Emory University, and the University of Utah will evaluate the use of a negative pressure dressing to decrease surgical site infections and therefore reduce antimicrobial use. They also will evaluate a machine learning model that can provide surgeons real-time decision support to prevent infections. The model could help surgeons determine if their patient is at risk for a surgical site infection and if the patient could benefit from negative pressure wound therapy, a prevention technique. Surgical site infections are common healthcare-associated infections that are treated with antibiotic use, which drives antibiotic resistance. Reducing these infections can improve patient outcomes and slow antibiotic resistance.
University of Illinois, College of Medicine, Chicago (Illinois, U.S.)
The University of Illinois, Chicago commits to researching the factors, including antibiotic use and infection control practices, that may contribute to the development and spread of resistance in the Dominican Republic. Researchers will perform a multi-site cross-sectional point-prevalence study to assess antibiotic use and resistance in acute care teaching hospitals, pharmaceutical dispensaries, and outpatient laboratories, as well as survey providers and consumers about their attitudes and perceptions of antibiotic use.
University of North Carolina Medical Center Antimicrobial Stewardship and Infection Prevention Programs (North Carolina, U.S.)
The University of North Carolina Medical Center’s Antimicrobial Stewardship and Infection Prevention Program commits to reducing the risk of antimicrobial resistance and C. difficile infections in its hospitals. The medical center is measuring a decrease in infection rates over time. The medical center program will decrease unnecessary broad-spectrum antibiotic use by prompting review and evaluation of antibiotic use after 48 hours (antibiotic time-out), enhanced stewardship activities, and infection prevention efforts focused on preventing hospital-acquired infections.
University of Washington Center for One Health Research (Washington, U.S.)
The University of Washington Center for One Health Research commits to collecting and analyzing One Health antimicrobial data stored in its Washington Integrated Surveillance for Antimicrobial Stewardship database. The database enables analysis of antibiotic resistance prevalence and trends in Washington and makes available data on different host species. The data also is used to support stewardship efforts in human, domesticated and wild animal, and environmental areas. The research center will create community antibiograms for humans and animals to inform prescribing practices and create antibiograms through sampling water and marine wildlife species in the Salish Sea to understand antibiotic resistance patterns.
University of Washington Tele-Antimicrobial Stewardship Program (Washington, U.S.)
The University of Washington Tele-Antimicrobial Stewardship Program (UW TASP) commits to working with Critical Access Hospitals and rural hospitals across the Pacific Northwest to reduce inappropriate antibiotic use via education, empowerment, and best practices. Working with the Washington State Department of Health, UW TASP will adopt and develop web-ready guidelines and practical toolkits to promote appropriate antibiotic use and reduce infections caused by drug-resistant germs.
Urgent Care Association of America (Illinois, U.S.)
As the largest urgent care professional association, with more than 3,300 member centers, the Urgent Care Association (UCA) will improve antibiotic use by co-hosting an Antibiotic Stewardship Summit in 2018 with the Antibiotic Resistance Action Center (ARAC); incorporating antibiotic stewardship as an essential component of any UCA-accredited organization’s quality plan (effective Jan. 1, 2019); and developing a Quality Commendation recognition in 2019 for urgent care organizations that demonstrate an exemplary commitment to appropriate antibiotic prescribing based on CDC’s Core Elements of Outpatient Antibiotic Stewardship framework.
U.S.-India Strategic Partnership Forum (India)
The U.S.–India Strategic Partnership Forum (USISPF) is a non-profit organization that seeks to strengthen the bilateral relationship between India and the U.S. Recognizing that antimicrobial resistance (AMR) is a critical issue for both countries, USISPF commits to: working with members and other relevant stakeholders to identify new business or public-private partnership models that can improve access to antibiotics and other antimicrobial products; helping to develop corporate social responsibility (CSR) initiatives on AMR to leverage India’s two percent CSR mandate for greater impact; providing guidance on best practices for preventing the release of antibiotics into the environment, and encouraging members to review their manufacturing and supply chains based on these standards; advocating for good stewardship of antibiotics in healthcare and agriculture; and working with the Department of Biotechnology and other partners to provide institutional support to biotechnology startups working on AMR.
Utah Department of Health (Utah, U.S.)
The Utah Department of Health commits to build upon its partnerships with the major healthcare systems in Utah to improve antibiotic stewardship efforts in 25 acute care hospitals and many outpatient facilities throughout Utah. Additionally, Utah will work with stewardship experts to improve antibiotic use and prescribing in long-term care through an education campaign and one-on-one collaboration with a subset of long-term care facilities in the state to improve policies and practices that encompass CDC’s Core Elements of Antibiotic Stewardship. Progress will be measured through a series of surveys, gap analysis, and working with the facilities to address identified gaps.
VCA, Inc. (California, U.S.)
VCA, Inc., the largest family of animal care providers in North America, comprised of over 900 general practice, specialty, and emergency veterinary hospitals, commits to promoting evidence-based prescribing of antimicrobials in its hospitals. VCA’s smart antibiotic use campaign will provide doctors with tools to make and implement informed antibiotic use decisions, educate pet owners on the importance of preventative medicine and appropriate antimicrobial use through excellent doctor communication and internal and digital education materials, and advance scientific knowledge in companion animal veterinary medicine.
Velox Biosystems (California, U.S.)
Velox Biosystems, a clinical diagnostics startup out of University of California – Irvine, is committed to applying their innovative detection technology to develop rapid, cost effective, and highly accurate tests and instruments to combat the rise of antibiotic resistance. Velox is currently developing a point-of-care rapid screening and phenotypic antibiotic susceptibility test to significantly improve the diagnostic accuracy of urinary tract infection (UTI) screening and enable healthcare providers to make objective antibiotic treatment decisions, thereby combating the vicious cycle of increasing resistance in UTIs.
Venus Remedies Limited (India)
Venus Remedies Limited, a research-driven Indian pharmaceutical company, commits to continued investment in its therapeutic pipeline of oral antibiotics for treatment of drug-resistant infections. The most advanced candidates are currently being evaluated in preclinical studies and data from the animal studies are expected in late 2019. Additionally, the company is committed to antibiotic stewardship through its campaign called PLEA (preserving life of existing antibiotics). Each year, PLEA volunteers reach out to more than 50,000 healthcare professionals in India and spread awareness on the judicious use of antibiotics and on maintaining a sanitary hospital ecology. Further, PLEA’s social media campaigns collectively reached out to over 1 million people globally in 2018 and Venus aims to expand its reach in 2019.
Vermont Department of Health (Vermont, U.S.)
The Vermont Department of Health is committed to working with each of the 15 acute care hospitals in Vermont to ensure that containment measures are enacted for all target resistant organisms within 24 hours of identification and reporting. Vermont’s list of target resistant organisms (reportable by law) includes carbapenem-resistant Enterobacterales, and more recently has been expanded to include carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter baumannii, and the often multidrug-resistant fungal pathogen Candida auris. Additionally, increased communications will strengthen linkages between Vermont’s clinical labs and the AR Lab Network to facilitate submission of isolates for detection of antibiotic resistance genes.
Vermont Oxford Network (Vermont, U.S.)
The Vermont Oxford Network (VON), comprised of more than 1,200 hospital members worldwide, commits to reducing antibiotic use for newborns in neonatal intensive care units by 45 percent by December 2022. This commitment to the AMR Challenge builds on VON’s successful commitment in 2015 to reduce antibiotic prescribing rates by 25 percent in newborns and NICUs; VON exceeded their target with an actual reduction of 34 percent across 187 centers from 38 states and 7 countries by 2017. With CDC and partnerships with hospitals, state health departments, health systems, and international participants, VON will employ a coordinated program of education, disciplined quality improvement, and focused audits. In partnership with the CDC, VON will collaborate to make key programs and materials publicly available including the Choosing Antibiotics in Newborns Wisely Toolkit, a universal training program and more than 200 data-driven quality improvement abstracts with tangible examples of measurable results.
Virginia Department of Health (Virginia, U.S.)
Virginia Department of Health (VDH) commits to addressing the threat of antibiotic resistance by increasing the number of onsite infection prevention and control assessments conducted at individual facilities by 50% by the end of 2021. Onsite assessments will be conducted for novel or targeted multidrug-resistant organisms and proactively at high-risk facilities. VDH commits to using outpatient antibiotic prescription claims data to make high-prescribers aware of available resources and initiate a dialogue on optimal antibiotic use. VDH also commits to collaborating with partner organizations in the Virginia Healthcare-Associated Infections Advisory Group to offer trainings to healthcare providers across practice settings.
Vizient, Inc. (Texas, U.S.)
Vizient, Inc., the largest member-driven health care performance improvement company in the U.S., serves more than half of the nation’s acute care hospitals. Vizient commits to working with more than 270 members in its Hospital Improvement Innovation Network (HIIN), funded by Centers for Medicare & Medicaid Services, to provide education, technical assistance, and evidence-based strategies to implement CDC’s Core Elements of Antibiotic Stewardship. Vizient will also work with HIIN to reduce the rate of healthcare-associated C. difficile by 20 percent by March 2019 and develop a dashboard to identify improvement opportunities in antibiotic utilization for populations that are at high risk for antibiotic resistance, such as oncology and transplant. Also in 2019, Vizient will establish an online catalog of appropriate antibiotic use best practices and resources for hospitals. Vizient will also publish the findings from a national research project on the impact of antibiotic allergies on antimicrobial use along with an accompanying toolkit to help guide hospitals and prescribers on assessment and medical management in patients with self-reported antibiotic allergies.
Walmart (Arkansas, U.S.)
Walmart US commits to improving responsible antibiotic use in supply chains in line with its position on antibiotic use in farm animals. With more than 5,000 stores and clubs nationwide, Walmart US will ask animal protein suppliers to report antibiotic use throughout their supply chain and will conduct blockchain projects, which will provide greater transparency about antibiotic use on the farm and throughout the supply chain, starting with pork in 2018.
Washington State Department of Health (Washington, U.S.)
The Washington State Department of Health commits to addressing the threat of antibiotic resistance through the Combating Antibiotic Resistant Bacteria Initiative to stop the emergence and transmission of antibiotic-resistant organisms. Activities include surveillance and lab testing for resistant organisms; optimizing infection prevention in all healthcare settings; encouraging appropriate communication of resistance threats to healthcare providers and facilities; promoting judicious use of antibiotics in all One Health settings; promoting appropriate use of vaccinations; and responding to and containing antibiotic-resistant threats. In addition, in 2019 Washington commits to releasing the One Health Antibiotic Stewardship Strategic Plan, and strengthening engagement with state commissions to gain a better understanding of issues such as facility oversight for outpatient clinics.
WaterAid (United Kingdom)
WaterAid commits to reducing antibiotic resistance by improving water, sanitation, and hygiene (WASH) in health facilities and communities in 28 low- and middle-income countries. WaterAid will also drive and ensure a multi-sectoral approach to address antibiotic resistance. WaterAid is focused on WASH as a fundamental and primary preventative measure to reduce the occurrence and spread of infections that lead to use, misuse, and overuse of antibiotics and that can drive resistance. Through a mix of advocacy and service delivery, WaterAid will collaborate with governments, health professionals, communities, and other partners to meet these commitments.
Water Engineers for the Americas and Africa (New Mexico, U.S.)
Water Engineers for the Americas and Africa (WEFTA) commits to implementing water and sanitation projects at six healthcare facilities in Bolivia, Mexico, Ethiopia, and Tanzania. WEFTA will partner with Daughters of Charity of St. Vincent de Paul, Village Health Partnership (VHP), Suma Jayma, ADSIS, and Sanitation & Water Action (SAWA) to support 20,000 people within these communities and are continually seeking additional partners.
Water for People (Colorado, U.S.)
Water For People, a non-profit working to promote clean drinking water and sanitation, commits to district-wide implementation within 30 districts across nine countries by 2030 to ensure every community, every school and clinic, and every household has access to reliable and sustainable water and sanitation services. Water For People serves the countries of Bolivia, Guatemala, Honduras, India, Malawi, Nicaragua, Peru, Rwanda, and Uganda. All healthcare facilities must develop sustainable safe drinking water, sanitation, and hygiene (WASH) services in collaboration with local government and health officials to build strong and well-funded systems that are locally managed and sustained.
Water Mission (South Carolina, U.S.)
Water Mission, a Christian engineering non-profit providing safe water in 55 developing countries and crisis areas, commits to continuing to provide technical guidance to safe drinking water, sanitation, and hygiene (WASH) implementers and WASH networks. Water Mission will continue to provide guidance on the construction of solar-piped water schemes for both last mile communities, refugee settlements, and institutions, including water quality testing and treatment. As part of Water Mission’s strategy, WASH in healthcare facilities will remain a priority in all 10 full-time country programs as well as disaster areas. Water Mission will continue to raise awareness about WASH best practices and the global water crisis and is committed to ongoing innovation and improving WASH best practices.
Wellbeing Foundation Africa (Nigeria)
Wellbeing Foundation Africa (WBFA) commits to training 500 healthcare workers and educating 35,000 pregnant and nursing mothers across Nigeria on hand and personal hygiene practices by 2020. WBFA also commits to advocating for improved safe drinking water, sanitation, and hygiene (WASH) structures in healthcare facilities in 2019 and 2020 to enable healthcare workers in Nigeria to perform their duties effectively.
Wellcome Trust (United Kingdom)
Wellcome Trust committed to a five-year, $225 million program of work to combat antimicrobial resistance, which combines investing in scientific research to support the development of new therapeutics and diagnostics, with efforts to support an effective response by national and global policy-makers. As a politically and financially independent global charitable foundation, we are committed to working with civil society, governments, and the private sector to find powerful solutions to the challenge of rising drug-resistant infections. The Call to Action conference is a key mechanism to bring these different groups together to share and celebrate best practice, and commit to tangible actions and continued progress.
West Virginia Bureau for Public Health (West Virginia, U.S.)
West Virginia Bureau for Public Health commits to conducting onsite visits to acute, long-term care, and outpatient facilities to assess infection control and antibiotic stewardship practices while providing guidance to strengthen programs. The Bureau partners with Charleston Area Medical Center to gather isolates and forward them to the U.S. CDC’s designated regional laboratory to better understand the increase of antibiotic resistance in West Virginia. The Bureau also commits to raising awareness on appropriate antibiotic use through education and increased communication to the public and providers.
Wildlife Center of Virginia (Virginia, U.S.)
The Wildlife Center of Virginia commits to completing a retrospective analysis of antibiotic resistance in wildlife that includes 10 years of data. In a wildlife rehabilitation setting, infections are commonly seen and treated empirically with antibiotics due to limited resources, access to specimens, and qualify laboratories. Current data show significant burden of resistant infections among birds, mammals, and reptiles. The data will be published by December 2020, highlighting common pathogens and resistance profiles for infections in these animals. This publication will inform appropriate antibiotic prescribing among wildlife veterinarians, and increase awareness of antibiotic resistance as a One Health issue.
Wisconsin Department of Health Services Division of Public Health (Wisconsin, U.S.)
The Wisconsin Department of Health Services (DHS) Division of Public Health commits to addressing the threat of antibiotic resistance through education and enhanced surveillance. DHS is developing a registry for healthcare providers to identify patients with previous positive carbapenemase-producing carbapenem-resistant Enterobacterales or other multidrug-resistant organism results. These germs can spread quickly and cause hard-to-treat infections. The registry supports faster implementation of infection control. DHS also supports improved detection by promoting CDC’s National Healthcare Safety Network reporting among hospitals and long-term care facilities and educating infection preventionists on core infection control and prevention best practices.
World Alliance Against Antibiotic Resistance (France)
The World Alliance Against Antibiotic Resistance (WAAAR), a global non-profit based in Paris, launched a new national initiative to improve antibiotic use in the fall of 2018. All Members of Parliament and scientific and medical society leaders received “An Implementation Manual, A Policy to Combat Antibiotic Resistance.” The English version was presented in May to the United Nations World Health Assembly and to the Global Antimicrobial Resistance (AMR) Research and Development Hub. Since 2015, WAAAR has edited AMR Control, a prestigious yearly publication featuring global World Health Organization, Centers for Disease Control and Prevention, Biomedical Advanced Research and Development Authority, World Bank, and national AMR leaders worldwide.
World Vision (Washington, U.S.)
World Vision, a humanitarian aid, development, and advocacy organization, and its partners commit to investing approximately $100 million from 2019-2021 in basic water, sanitation, and hygiene services in 800 rural healthcare facilities, serving an estimated 7.2 million people. The work will be done in 35 countries where World Vision focuses on safe drinking water, sanitation, and hygiene (WASH) efforts, focusing on Ghana, Mali, Niger, Rwanda, Uganda, Tanzania, Malawi, Zambia, and Zimbabwe. Key partners include charity: water, Grundfos, CDC, Conrad N. Hilton Foundation, the Dornsife Family, Golf Fore Africa, Midmark Corporation, Robert and Laura Abernathy, the Water Institute at the University of North Carolina-Chapel Hill, and Wells Bring Hope.
Wyoming Department of Health (Wyoming, U.S.)
The Wyoming Department of Health commits to promoting the appropriate use of antibiotics and vaccinations in hospitals and nursing homes. The department will improve infection prevention in all healthcare settings. It will encourage appropriate communication of resistance threats to healthcare providers and facilities. The department will provide surveillance and conduct lab testing for resistant organisms. Additionally, it will respond to and contain antibiotic-resistant threats by working with statewide partners, its Quality Innovation Network-Quality Improvement Organization, hospital associations, healthcare facilities, and various health department programs. The department will measure impact by the improvement of cluster response and disease rates.
Yum! Brands (Kentucky, U.S.)
Yum! Brands, parent company of KFC, Pizza Hut, and Taco Bell, commits to continuing good antibiotic stewardship by removing all antibiotics important to human medicine from chickens used for wings in their U.S. based Pizza Hut restaurants by 2022. Yum! Brands will also reduce the use of antibiotics important to human health by 25% in its Taco Bell beef supply chain by 2025. KFC, Pizza Hut, and Taco Bell in the U.S. have already met public commitments to remove antibiotics important to human medicine from their poultry supplies.
Zanmi Lasante (Haiti)
Zanmi Lasante, known as Partners In Health in Haiti, commits to ensuring that its supported healthcare facilities are in line with national guidelines required by the Haitian government and international standards for access to water, sanitation, and hygienic practices among its medical personnel. Zanmi Lasante commits that all efforts to ensure patient safety and security in accessing clean water and sanitation services remain a priority of the organization at all times.
Zoetis (New Jersey, U.S.)
Zoetis, a global animal health company, commits to continue advocating for a One Health approach to the responsible use of antibiotics. Zoetis commits to promoting the involvement of veterinary professionals in antibiotic stewardship, expanding access to veterinary care worldwide and supporting broader use of vaccines and modern animal husbandry and biosecurity practices to help prevent disease. Zoetis is working to develop veterinary-only antibiotics and antibiotic alternatives; novel vaccine technologies; diagnostic tests; sensors sending and analyzing digital information to predict and detect underlying signs of disease; and genetic tests for wellness traits. Zoetis will continue its more than two-decade investment in surveillance for antibiotic resistance in germs that threaten the health of livestock and companion animals to help preserve the efficacy of antibiotics.