Combating the Threat of Antimicrobial-Resistant Gonorrhea
Antimicrobial-Resistant Gonorrhea: A Public Health Threat
- Gonorrhea is the second most commonly reported bacterial sexually transmitted infection in the United States.
- There are more than 500,000 reported cases of gonorrhea per year, yet CDC estimates 1.6 million new infections may actually occur each year.
- About half of all infections each year are resistant to at least one antibiotic.
- Today, the U.S. has just one recommended gonorrhea treatment option remaining.
The National Strategy
In 2013, CDC released Antibiotic Resistance Threats in the United States, 2013, the first report to look at the burden and threats posed by antibiotic resistance on human health. This report named resistant gonorrhea among the three most urgent threats of its kind in the country. One year later, the President released an Executive Order and the White House developed the National Strategy to Combat Antibiotic-Resistant Bacteria (CARB), both of which call for the prevention, detection, and innovation against resistance.
In March 2015, the White House released the five-year National Action Plan for CARB, which outlined steps for implementing the National Strategy. This Plan – The National Action Plan for Combating Antibiotic-Resistant Bacteria (CARB), 2020-2025 – was updated in 2020 to present coordinated, strategic actions that the U.S. government will take in the next five years to improve the health and well-being of all Americans by changing the course of antimicrobial resistance. To meet these national goals, and through federal funding for CARB, CDC’s Division of STD Prevention (DSTDP) is supporting a number of new and continuing activities that aim to slow the development of resistant gonorrhea and prevent its spread. Gonorrhea control in the U.S. relies on our ability to detect and treat each case of gonorrhea quickly and effectively with the right antibiotic; because of this, DSTDP is taking a multipronged, capacity-building approach to tackle the problem.
Determining the Best Way to Treat Gonorrhea
In large part, DSTDP makes decisions about national treatment recommendations using data from the Gonococcal Isolate Surveillance Project (GISP). Gonorrhea specimens from men with symptoms of urethritis in 26 STD clinics in selected U.S. cities are collected each month for culture and sent to their local laboratory for isolation of the bacteria. Regional laboratories in the AR Lab Network then test these isolates for resistance to the different antibiotics that are, or were, used to treat gonorrhea. All clinical, epidemiologic, and laboratory results are then sent to CDC and used to determine the most effective treatment to recommend for gonorrhea, based on nationwide susceptibility and resistance patterns and trends.
Ensuring Individuals Receive Recommended Treatment
To make sure individuals with gonorrhea are receiving CDC’s recommended treatment, DSTDP monitors a representative sample of reported gonorrhea cases from the STD Surveillance Network (SSuN). SSuN is a sentinel network of 10 geographically diverse state and local health department STD prevention programs. Directly-funded STD programs also receive support to monitor gonorrhea treatment practices in their jurisdiction.
Improving Laboratory Work Central to the Threat Response
CDC’s AR Solutions Initiative funds the Antimicrobial Resistance Laboratory Network (AR Lab Network)—a network of public health laboratories that provide cutting-edge antimicrobial resistance laboratory testing for existing and emerging threats. Since gonorrhea is an important part of CARB activities, four regional labs in the AR Lab Network are funded to build robust capacity for culture-based antibiotic susceptibility testing and genomic sequencing. These labs process up to 20,000 isolates per year from GISP and rapid detection and response activities. The public health data generated by the AR Lab Network helps DSTDP better monitor resistance and inform gonorrhea treatment recommendations and prevention interventions. A select number of isolates tested by the AR Lab Network are archived in the CDC and FDA Antimicrobial Resistance Isolate Bank. These isolates can be used for developing diagnostics, antibiotics, and vaccines for gonorrhea, as well as support other future antimicrobial-resistant gonorrhea studies.
Preparing to Rapidly Detect and Respond to Resistant Gonorrhea Strains
CDC’s AR Solutions Initiative is also supporting the multisite activity, Gonorrhea Rapid Detection and Response, which will develop and strengthen local and state health department epidemiological, laboratory, and informatics capacity to more rapidly detect, and quickly and effectively respond to resistance through network analysis of transmission dynamics and novel interventions. The AR Investment Map shows CDC’s key investments to combat resistance, including resistant gonorrhea, across the nation.
This work is done in many ways, as illustrated by the following key examples:
(1) Hiring and training state and local public health personnel so they have better tools and systems to respond to emerging threats;
(2) Increasing epidemiological investigations of gonorrhea cases and their sexual and social networks to help local jurisdictions better understand gonorrhea-related transmission dynamics in their area;
(3) Expanding the use of culture beyond male urethritis in STD clinics, so that extragenital specimens and specimens from women with gonorrhea are routinely collected, as well as test-of-cure specimens as needed;
(4) Exploring different ways to expand gonorrhea culture to sexual and social networks, populations, and funded jurisdictions where nucleic acid amplification tests (NAATs) without antimicrobial susceptibility testing markers are the current gonorrhea diagnostic testing method;
(5) Establishing Etest capacity as a rapid way to detect susceptibility and resistance until novel rapid molecular tests are in place; and
(6) Rapidly providing lab results on resistance to providers and public health personnel to quickly identify, treat, and stop the spread of resistant gonorrhea strains.
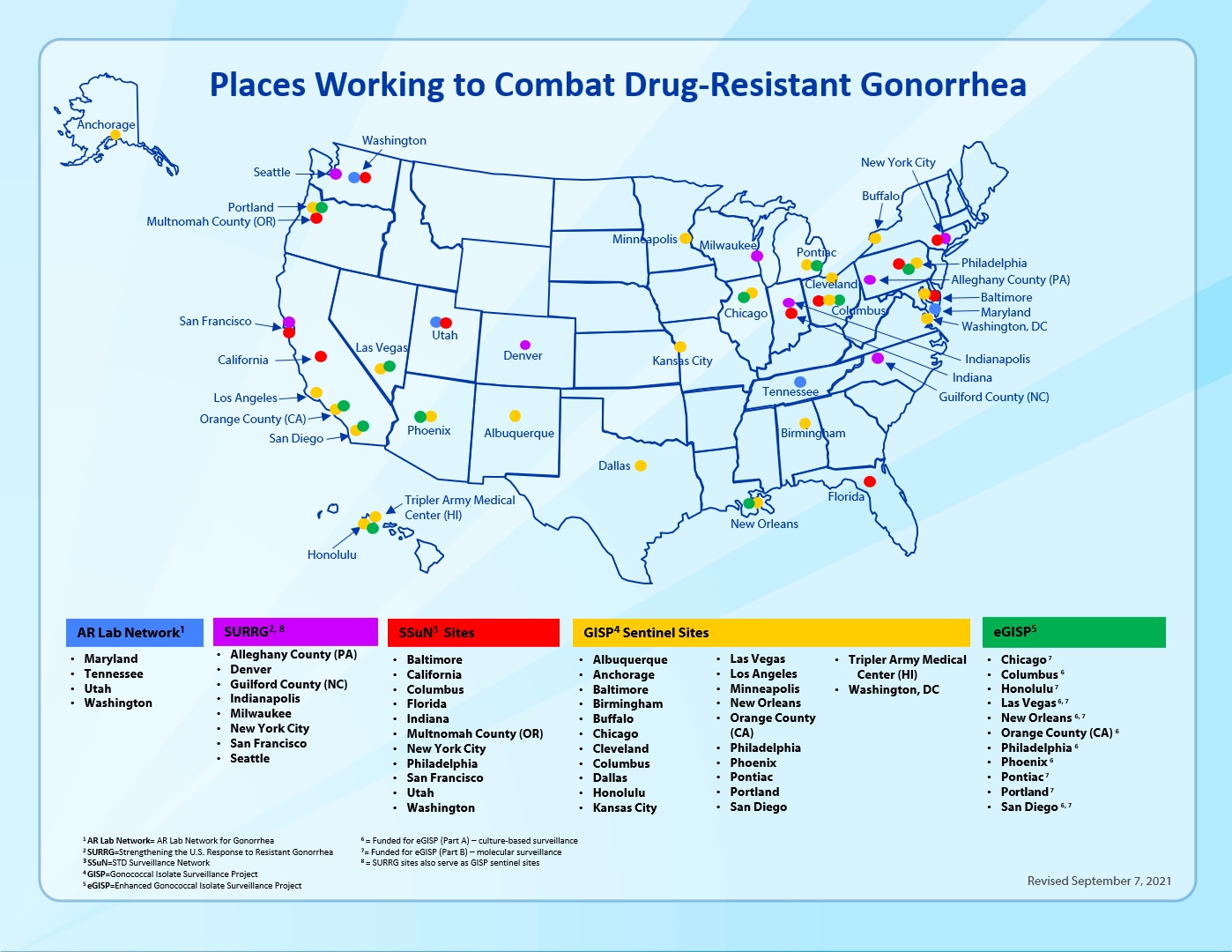
For 30 years, CDC has monitored antimicrobial-resistant gonorrhea trends to help ensure that the recommended drug treatment for gonorrhea is working. With support from Congress, we arm state and local health departments with the tools they need to stay a step ahead of resistant gonorrhea – one of the nation’s most urgent public health threats. We also work together with health departments to enhance their capacity to monitor and test for resistant gonorrhea and develop rapid response strategies if resistance is detected. See below for a brief overview of current activities.

Established in 1986, the Gonococcal Isolate Surveillance Project (GISP) monitors U.S. antimicrobial resistance trends in gonorrhea. Through the collaborative effort of selected STD clinics and their local laboratories, regional laboratories, and CDC, GISP’s collected data helps ensure patients with gonorrhea receive the right antibiotic treatment. GISP monitors antimicrobial susceptibility of approximately 5,000 male gonococcal urethritis cases seen in 27 STD clinics.
The enhanced Gonococcal Isolate Surveillance Project (eGISP) strengthens surveillance of resistant gonorrhea and increases state and local capacity to detect and monitor it. In select STD clinics, eGISP not only collects samples from men with gonococcal urethritis but also from women and from extragenital sites. These specimens are sent to regional laboratories for susceptibility testing.

The STD Surveillance Network (SSuN) is a collaborative network of state, county and/or city health departments funded by CDC to conduct sentinel and enhanced STD surveillance activities. The purpose of SSuN is to improve the capacity of national, state and local STD programs to detect, monitor, and respond to trends in STDs through enhanced collection, reporting, analysis, visualization, and interpretation of disease information.

Strengthening the United States Response to Resistant Gonorrhea (SURRG) began in 2016 with three goals: 1) enhance domestic gonorrhea surveillance and infrastructure; 2) build capacity for rapid detection and response to resistant gonorrhea through increased culturing and local antibiotic susceptibility testing; and 3) rapid field investigation to stop the spread of resistant infections. The project also aims to gain a better understanding of the epidemiological factors contributing to resistant gonorrhea. Nine jurisdictions collect and analyze data, helping guide national recommendations for the public health response to resistant gonorrhea.

The Antimicrobial Resistance Laboratory Network (AR Lab Network) is a network of public health laboratories equipped to respond to emerging health threats and provide cutting-edge resistance laboratory support. Since gonorrhea is an important part of these activities, four regional labs in the AR Lab Network receive funding for culture-based antimicrobial susceptibility testing and genomic sequencing.