US Zika Pregnancy and Infant Registry
The US Zika Pregnancy and Infant Registry (USZPIR) is a collaborative and innovative system to learn about Zika virus infection during pregnancy and after birth. Information from the USZPIR is used to make recommendations for healthcare providers caring for families affected by Zika virus and understand the spectrum of outcomes among infants with possible congenital Zika exposure.
How It Works
Watch to learn how CDC, health departments, and healthcare providers track Zika virus infection to protect mothers and babies.
To share this image, click on an icon below.
Social_round_facebook

Goals of the USZPIR
The US Zika Pregnancy and Infant Registry collects information about pregnant people in the United States with laboratory evidence of possible Zika virus infection and their infants. This includes tracking pregnant people who completed their pregnancies from December 1, 2015 to March 31, 2018.
CDC scientists use this information to identify adverse pregnancy and infant outcomes in people with Zika virus infection. Information from the USZPIR is also used to answer important questions about infants born to mothers infected with Zika virus during pregnancy. Currently, the total range of effects of Zika virus infection on these infants remain unknown. This is why it is important to collect medical information about the infant, even if he or she appears healthy at birth.
The USZPIR aims to answer questions about Zika and to provide important information that can help CDC researchers make recommendations for clinicians who are caring for pregnant people and infants. Information from the USZPIR might also be used to help efforts to prevent Zika virus infection during pregnancy.
Who Is Included
Any pregnant person or infant with laboratory evidence of possible Zika virus infection can be enrolled in the USZPIR if the pregnancy was completed from December 1, 2015 to March 31, 2018. This includes pregnant people who were infected with Zika up to 6 weeks before they became pregnant, during pregnancy, or at the time they delivered their baby. If a pregnant person is enrolled in the USZPIR, her infant will also be enrolled. Similarly, if an infant tests positive for Zika virus infection at birth and is enrolled in the USZPIR, their mother will also be included.
Pregnant people and infants who tested positive for Zika virus infection can be enrolled regardless of if they have symptoms. The USZPIR includes all 50 states, the District of Columbia, and the following jurisdictions: American Samoa, Chicago, Federated States of Micronesia, Guam, Houston, Los Angeles County, New York City, Philadelphia, Puerto Rico, Northern Mariana Islands, Republic of the Marshall Islands, Republic of Palau, and US Virgin Islands.
Participation
Health Departments
The success of the USZPIR depended on the voluntary collaboration of state, tribal, local, and territorial health departments. These health departments participate by
- Identifying pregnant people and infants for Zika virus testing
- Coordinating Zika testing of laboratory samples
- Reporting information about pregnant people and infants with laboratory evidence of possible Zika virus infection
- Collecting medical information about eligible pregnant people and their infants
- Working with CDC to determine state-specific methods for collecting and sharing data
From August 2016 through July 2018, 61 states, territories, freely associated states, and local health departments received Zika funding to support the US Zika Pregnancy and Infant Registry. CDC continues to support data collection efforts by jurisdictions. Data collected through the USZPIR help CDC scientists to learn more about the long-term health outcomes of infants affected by Zika during pregnancy.
Jurisdictions Funded for the U.S. Zika Pregnancy and Infant Registry in August 2016 and Contractual Field Staff Placement Sites (August 2016-July 2018)
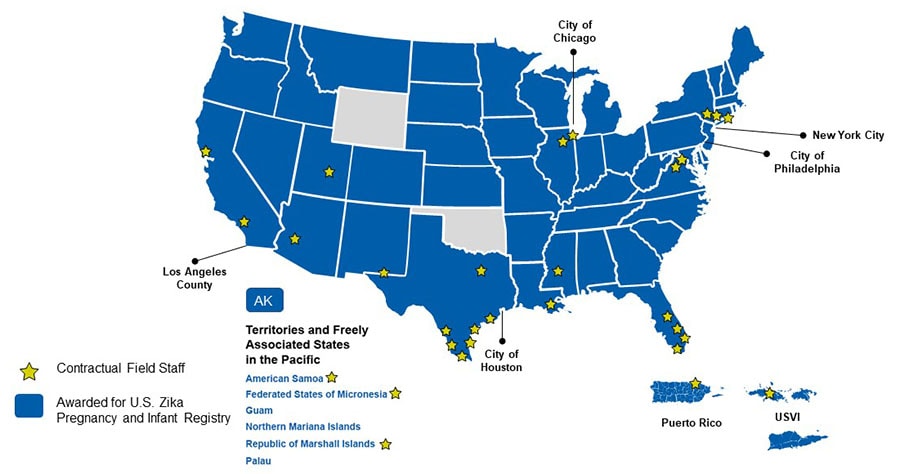
*Jurisdictions and partners were supported through either a cooperative agreement or contractual mechanism. Jurisdictional cooperative agreements were funded through the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases (ELC).
Doctors & Nurses
Healthcare providers participate in the USZPIR by helping to report medical information of eligible pregnant people and infants to their health department. Healthcare providers should report information on eligible pregnant people and infants to their state, local, tribal, or territorial health departments. Eligible pregnant people and infants include those that meet USZPIR criteria and have completed their pregnancies from December 1, 2015 to March 31, 2018. Pregnancies with possible Zika virus infection that are ongoing after March 31, 2018, will not be included in analyses of the USZPIR data. However, health departments may continue to securely send follow-up data to CDC USZPIR staff.
The data gathered through the USZPIR will help scientists understand the effects of Zika virus in the United States, healthcare providers appropriately care for families affected by Zika virus, and CDC continue to update guidance for the care of pregnant people with Zika virus infection and their infants.
The USZPIR Process
Data Collection
Various methods (e.g., medical record abstraction, linking to vital records) are used to collect information for the US Zika Pregnancy and Infant Registry.
Data collection begins when a pregnant person tests positive for Zika virus infection. A pregnant person can test positive for Zika at any point during pregnancy or at delivery. Once a woman tests positive, healthcare providers collect medical information at the following time periods (regardless of whether symptoms appear or not):
- Initial identification of Zika virus infection in a pregnant person or infant
- During pregnancy
- At delivery
- At the baby’s
- 2 month follow-up visit
- 6 month follow-up visit
- 12 month follow-up visit
- 18 month follow-up visit
- 24 month follow-up visit
- 30 month follow-up visit
- 36 month follow-up visit
*Puerto Rico also collects data at the baby’s 9 month, 42 month, 48 month, 54 month, 60 month follow-up visits. US Virgin Islands also collects data at the baby’s 48 month and 60 month follow-up visits.
Communicating Findings
When the Zika outbreak began, there were many unknowns. Since then, we have learned a lot about Zika virus infection during pregnancy because of the rich data that health departments collected. This data has helped us to answer many questions about how Zika virus can affect babies.
Protection of Medical Information
CDC takes the protection of confidential and sensitive information very seriously. CDC follows strict standards to ensure that the privacy of people whose health information we collect is protected. Information in the US Zika Pregnancy and Infant Registry is collected in a way that ensures it is safeguarded against unauthorized disclosure. CDC will not release any identifiable data.
CDC requests the collection of clinical information in identifiable form as a public health authority. As defined in the Health Insurance Portability and Accountability Act (HIPAA) and the Standards for Privacy of Individually Identifiable Health Information regulations (“Privacy Rule”), healthcare providers may disclose protected health information without patient authorization to a public health authority if the purpose is preventing or controlling disease.
An additional safeguard is that the medical information in the US Zika Pregnancy and Infant Registry and Zika Birth Defects Surveillance system is protected by an Assurance of Confidentiality. CDC has determined that both systems are non-research public health surveillance activities.