Preparing Solutions for Qualitative Fit Testing from Available Chemicals
Before a model of tight-fitting respirator can be used in the workplace, a qualitative or quantitative fit test must be performed to confirm that the respirator forms a tight seal against the user’s face, ensuring that the user will receive the expected level of protection. A qualitative fit test (QLFT) is a pass/fail test to assess respirator fit based on a worker’s response to a test agent. More information on fit testing is provided at: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/respsource3fittest.html
The agents used in qualitative fit testing are usually available commercially in convenient, ready-to-use packaging that contains premade dilutions of the reagents. This will often include all the consumables needed to perform the test (stirrer, absorbent paper, nebulizer, etc.). When these ready-to-use packages are not available, the QLFT can be performed by using the required solutions prepared from commercially available reagents.
Approved Qualitative Fit Testing Agents
The Occupational Safety and Health Administration (OSHA)-accepted fit test protocols require that United States Pharmacopeia (USP)-grade reagents be used in the preparation of all of the required solutions. Instructions to make the OSHA-approved fit test and threshold check solutions for Saccharin Solution Aerosol Protocol, Bitrex® (Denatonium Benzoate) Solution Aerosol Qualitative Fit Test Protocol, and Isoamyl Acetate Protocol are provided below. Information on irritant smoke is not provided here.
The OSHA-accepted fit test protocols provide complete instructions for conducting QLFTs with these agents. Test agents are used in a similar way in each of the protocols: a worker is initially tested without wearing the respirator to ensure that they are able to detect the agent by smell or taste. This is performed using a threshold check solution that is a dilution of the fit test solution. After ensuring the worker can smell or taste the agent, the worker puts on the respirator and fit testing is performed using the fit test solution. If the worker is able to smell or taste the agent while wearing the respirator, the fit test fails and the respirator may need to be redonned or repositioned and retested. If a fit test continues to fail, the worker will need to be fitted with a different respirator.
Preparation of Saccharin Sodium Fit Test and Threshold Check Solutions
The instructions provided below will produce the two solutions needed to conduct qualitative respirator fit testing in accordance with OSHA’s Respiratory Protection Standard. The “fit test solution” developed from these instructions uses saccharin sodium dihydrate, not sodium saccharin anhydrous, and is equivalent to the “fit test solution” listed at step(b)(5) of the Saccharin Solution Aerosol Protocol from OSHA. The “threshold check solution” developed from these instructions is equivalent to the “threshold check solution” listed as step(a)(5) of the Saccharin Solution Aerosol Protocol from OSHA. The instructions make about 100mL of fit test solution and 100mL of threshold check solution. This is expected to be enough to complete about 30‒70 qualitative fit tests.
Ingredients:
- USP saccharine sodium dihydrate (CAS No. 6155‒57‒3) is commercially available generally in 500g packaging. Do not substitute other forms of saccharin or use any other sweetener. These instructions are specific for sodium saccharin dihydrate.
- Distilled water (CAS No.7732‒18‒5) is commercially available generally in 1L bottles.
Do NOT use tap water, spring water, or mineral water.
Items Needed:
- Two 100mL glass containers with lids
- One 150mL glass container with lid
- Transfer pipette or a 100mL syringe
- Scale (capable of measuring grams)
- Eye dropper or ml syringe
- Spoon or stirrer
- Thermometer
- Hot plate (or similar tool to apply heat from the bottom)
Preparation:
- Label container 1 (one of the two 100mL containers) as “Fit Test Solution”.
- In container 2 (the 150mL container), add 106g of saccharine sodium dihydrate to 110mL of distilled water at room temperature to obtain a saturated solution. Close the container.
- Gently swirl the solution. If necessary, apply heat (the solution should not exceed 45°C or 113°F) to help dissolve the saccharin sodium dihydrate.
- If powder fully dissolves, transfer contents of container 2 into container 1 labelled “Fit Test Solution”. This is your final fit test solution.
- If a sediment is visible at the bottom container 2, allow the solution to rest for a couple of minutes. Then transfer the liquid portion of the solution in container 2 into the container 1 labelled “Fit Test Solution” by using a pipette or syringe. Pay attention not to carry over the solid salt. This is your final Fit Test Solution. About 5‒10ml of the solution at the bottom of container 2 can be left behind and discarded.
- Label container 3 (the last 100mL container) as “Threshold Check Solution”.
- In container 3, add 1ml (or 20 drops) of the Fit Test Solution to 99mL of distilled water.
- Mix vigorously. This is your final threshold check solution.
Preparation of Denatonium Benzoate Fit Test and Threshold Check Solutions
The instructions provided below will produce the two solutions needed to conduct qualitative respirator fit testing in accordance with OSHA’s Respiratory Protection Standard. These instructions make about 200mL of fit test solution and 100mL of threshold check solution. This is expected to be enough to complete about 30‒70 qualitative fit tests. The number of qualitative fit tests that can be performed depends on a host of factors.
Administering this fit test requires the use of two nebulizers (one for the threshold check solution and one for the fit test solution) with 1mL of solution in each. Several fit tests can be performed using these nebulizers, but the nebulizers should be thoroughly rinsed with water, shaken dry, and refilled at least every four hours as listed in step(a)(14) of the Bitrex® (Denatonium Benzoate) Solution Aerosol Fit Test Protocol from OSHA.
This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200). Creating a mixture of Denatonium Benzoate in a secondary container requires a Secondary Label that includes the identity of the hazardous chemical within the container and the hazards the components present. For convenience, Figure 1 provides suggested labels.
Ingredients:
- Denatonium Benzoate (CAS No. 3734-33-6) is commercially available generally in 5-15g packaging.
- Distilled water (CAS No.7732‒18‒5) is commercially available generally in 1L bottles.
- Sodium Chloride (CAS No. 7647-14-5) is commercially available generally in 500g packaging.
Do NOT use tap water, spring water, or mineral water.
Items Needed:
- One 500mL glass container
- Two 200mL glass containers with lids
- Transfer pipette or a 100mL syringe
- Scale (capable of measuring milligrams)
- Spoon or stirrer
- Thermometer
- Hot plate (or similar tool to apply heat from the bottom)
Preparation:
Preparation of 5% NaCl Solution
- In container 1 (the 500mL container), add 17.51g of NaCl to 350 mL of distilled water and stir well ensuring that all salts are dissolved.
This solution is used as an intermediate solution for the preparation of the final fit test solution and the final threshold check solution.
Preparation of Fit Test Solution
- Label container 2 (one of the two 200mL containers) with the Denatonium Benzoate Fit Test Solution label, Figure 1 (left)
- Add 337.5mg of Denatonium Benzoate to 200 mL of the previously prepared 5% NaCl solution.
Stir well, ensuring that all of the solids dissolve. If necessary, apply gentle heat (the solution should not exceed 45°C or 113°F) while stirring to help dissolve the solids. This your final fit test solution.
Preparation of Threshold Check Solution
- Label container 3 (the last 200mL container) with the Denatonium Benzoate Threshold Check Solution label, Figure 1 (right)
- Add 13.5mg of Denatonium Benzoate to 100 mL of the previously prepared 5% NaCl Solution. Close the lid.
- Gently swirl the solution. If necessary, apply heat (the solution should not exceed 45°C or 113°F) to help dissolve the salt. This is your final threshold check solution.
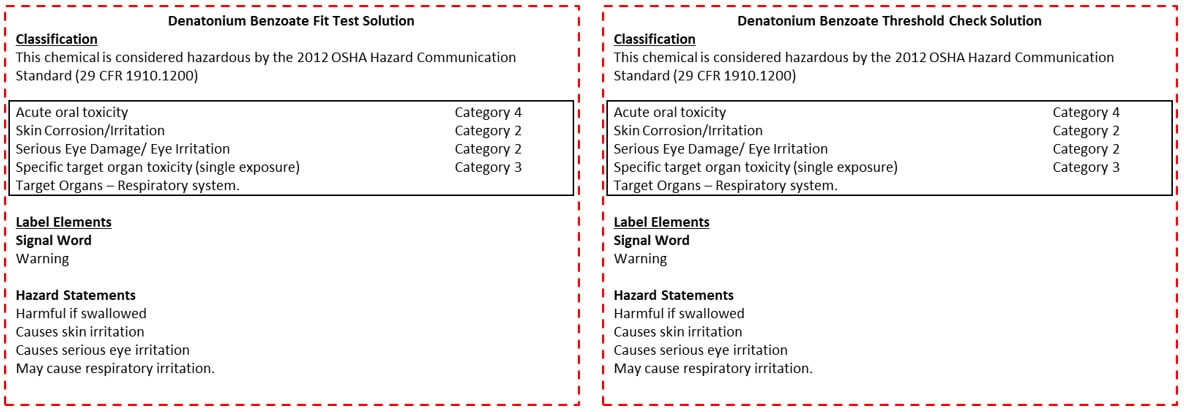
Figure 1: Denatonium Benzoate Solutions Secondary Labels. The label on the left should be printed and attached to container 2 for the Denatonium Benzoate fit test solution. The label on the right should be printed and attached to container 3 for the Denatonium Benzoate threshold check solution. Image
NOTE: label originated from the Fisher Scientific SDS for denatonium benzoate
Preparation of Isoamyl Acetate (IAA) Odor and Test Blank Solutions
This is not to be used for filtering facepiece respirators such as N95® or P100®
The isoamyl acetate protocol, commonly referred to as banana oil, is not appropriate to use for the qualitative fit testing of particulate respirators, including any filtering facepiece respirators (e.g., N95®, P100®). Elastomeric respirators fit tested with the IAA protocol, must be equipped with an organic vapor filter/cartridge.
The instructions provided below will produce the solutions needed to conduct qualitative respirator fit testing in accordance with OSHA’s Respiratory Protection Standard. The solution used to determine the worker’s ability to detect the odor of the IAA is named “odor test solution” and is made by diluting the pure reagents as described below. The pure reagent (straight from its original container) is applied to a paper towel (or similar porous material) during the qualitative fit test.
Ingredients:
- Pure isoamyl acetate also called isopentyl acetate (CAS No. 123‒92‒2) is commercially available in liquid form under either name.
- Distilled water (CAS No. 7732‒18‒5) is commercially available generally in 1L bottles.
Do NOT use tap water, spring water, or mineral water. -
Items Needed:
- Three 1L glass jars with metal lids
- 1L graduated cylinder
- Transfer pipette
- Eye dropper or a syringe
Preparation:
- Label container 1 as “stock solution”
- In container 1 add 1mL isoamyl acetate to 800mL of distilled water. Close the lid and shake for 30 seconds. This is the stock solution and new solutions should be prepared at least weekly. Old solutions should be disposed of following requirements at your location.
- Label container 2 as “odor test solution”. Place 0.4mL of the stock solution into 500mL of distilled water. Close the lid and shake for 30 seconds. Allow the solution to stand for two to three minutes so that the isoamyl acetate concentration above the liquid may reach equilibrium. This solution is the odor test solution and should be used for only one day.
- Label container 3 as “test blank”
- Add 500mL of distilled water in container 3. This is the test blank, that together with the odor test solution is used to assess the individual’s ability to detect the smell of the isoamyl acetate (banana oil).
Storage Instructions for Fit Test and Threshold Check Solutions
- Store the solution in sealed containers at room temperature.
- If crystals form in the solution, place the sealed container in warm water and shake/swirl until the crystals re-dissolve.
- If the solution appears cloudy or dirty, discard as it may have been contaminated.
- Saccharin Sodium and Denatonium Benzoate fit test and threshold check solutions should be discarded every 30 days. Isoamyl Acetate odor test and stock solutions should be discarded weekly.
Acknowledgments
This document was prepared by Michelangelo Di Giuseppe, Jonisha Pollard, William King, and Evanly Vo of the National Personal Protective Technology Laboratory, National Institute for Occupational Safety and Health.
References
NIOSH [2018]. Healthcare respiratory protection resources. Pittsburgh, PA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, National Personal Protective Technology Laboratory. https://www.cdc.gov/niosh/npptl/hospresptoolkit/fittesting.html
OSHA [2004]. Appendix A to §1910.134—Fit testing procedures (mandatory). Part I. OSHA―Accepted fit test protocols. A. Fit testing procedures—general requirements. Washington, DC: Department of Labor, Occupational Safety and Health Administration. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
N95 is a certification mark of the U.S. Department of Health and Human Services (HHS) registered in the United States and several international jurisdictions. P100 is a certification mark of the U.S. Department of Health and Human Services (HHS) registered in the United States. Bitrex is a registered trademark of Macfarlan Smith Ltd.