NCIRD National Surveillance Materials and Resources
- VPD Morbidity in the US
- Surveillance in NNDSS
- VPD Surveillance Manual
- Surveillance Indicators
- VPD Reference Centers
- Coordination and Enhancement of Vaccine-Preventable and Respiratory Disease Surveillance
- NNDSS Modernization and the Data Modernization Initiative
- Worksheets for Disease/Condition Surveillance
- Vaccine Preventable Diseases
- Non-Vaccine Preventable Diseases
- Resources and Links
Vaccine–Preventable Disease Morbidity in the United States
A comparison of the morbidity and mortality before and after widespread implementation of national vaccine recommendations for 13 vaccine-preventable diseases for which recommendations were in place prior to 2005.
Manuscript
Roush SW, Murphy TV, Vaccine-Preventable Disease Table Working Group a. Historical Comparisons of Morbidity and Mortality for Vaccine-Preventable Diseases in the United States. JAMA. 2007; 298(19): 2155-2163. doi:10.1001/jama.298.18.2155.
Presentation Slides
These slides are updated annually to provide vaccine-preventable disease surveillance data for
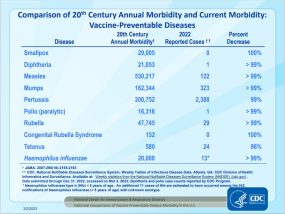
Comparison of 20th Century Annual Morbidity and Current Morbidity: Vaccine-Preventable Diseases (slide one [1 page]) (2023 Week 52 NNDSS provisional data as of 12/31/23) updated 2/14/24
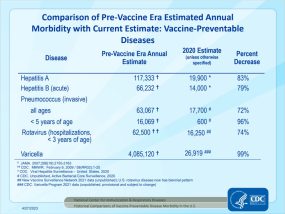
Comparison of Pre-Vaccine Era Estimated Annual Morbidity with Current Estimate: Vaccine-Preventable Diseases (slide two [1 page]) updated 3/20/2024
References
- National Notifiable Infectious Diseases Surveillance System, Notifiable Infectious Diseases Tables, Week 52. (2023 Provisional Data) Unpublished. Atlanta, GA. CDC Division of Health Informatics and Surveillance, 2023. Accessed on January 24, 2024.
- Prevention of Rotavirus Gastroenteritis among Infants and Children Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2009; 58 (RR02); 1-25.

For educational materials, see Surveillance Trainings & Videos.
Surveillance of NCIRD Diseases and Conditions in the National Notifiable Diseases Surveillance System (NNDSS)
In cooperation with jurisdiction/state health departments, the National Center for Immunization and Respiratory Diseases (NCIRD) coordinates for 23 diseases and conditions for which data comes through NNDSS, with 20 nationally notifiable and 3 under standardized surveillance, including vaccine-preventable diseases (VPDs) and other respiratory diseases and conditions. CDC is notified by jurisdiction/state health departments of cases of diseases and conditions under national surveillance, as designated by the Council of State and Territorial Epidemiologists (CSTE). NCIRD encourages jurisdiction/state health departments to submit provisional data through NNDSS before completing case investigations; however, cases are included for publication as described in the NNDSS event code list (case status print criteria) approved by CSTE.
CDC publishes NNDSS data weekly in the MMWR and/or CDC WONDER, and yearly in the Annual Summary of Notifiable Diseases. NNDSS data, together with data collected through supplemental surveillance activities, are analyzed by NCIRD staff and are disseminated through surveillance reports, journal manuscripts, CDC WONDER, MMWR Surveillance Summaries, and other publications.
NCIRD subject matter experts coordinate surveillance for 20 nationally notifiable conditions, specifically:
Manual for the Surveillance of Vaccine-Preventable Diseases
The Manual for the Surveillance of Vaccine-Preventable Diseases provides current guidelines for those directly involved in surveillance of VPDs, especially personnel at the local and state/territorial (jurisdiction) health departments. For each of the VPDs, the manual includes a chapter describing the importance of rapid case identification; the importance of surveillance; disease reduction goals; case definitions (including clinical description and case classifications); epidemiologically important data to be collected during case investigation; activities for enhancing surveillance; activities for case investigation; and activities for outbreak control. Other chapters include information on surveillance indicators; surveillance data analyses; enhancing surveillance; laboratory support for surveillance; and reporting adverse events following vaccination. In addition, the manual includes a section reserved for the insertion of jurisdiction-specific guidance for VPD surveillance, as well as extensive appendices.
Surveillance Indicators
The purpose of vaccine-preventable disease surveillance indicators in the United States is to ensure adequate performance of the essential components of surveillance and case investigation, and to identify components of each that need improvement. Surveillance indicators for selected vaccine-preventable diseases were proposed by CDC and approved by CSTE in 1994. Since then, the indicators have continued to evolve to maximize their usefulness. The CDC currently monitors indicators for measles, mumps, rubella, Haemophilus influenzae, pertussis, meningococcal disease, invasive pneumococcal disease and varicella on a regular basis.
APHL/CDC Vaccine-Preventable Disease Reference Centers
The VPD Reference Centers are four public health laboratories that work closely with the Association of Public Health Laboratories (APHL) and CDC to provide quality testing free of charge to other public health laboratories and departments. The VPD Reference Centers are located in California, Minnesota, New York and Wisconsin, and provide testing for measles, mumps, rubella, varicella-zoster, B. pertussis, S. pneumoniae, N. meningitidis, and H. influenzae. For additional information about the testing available at the VPD Reference Centers see APHL’s vaccine-preventable diseases program.
Coordination and Enhancement of Vaccine-Preventable and Respiratory Disease Surveillance
Project support is provided through the Epidemiology and Laboratory Capacity for Infectious Disease (ELC) Cooperative Agreement (CoAg) to enhance vaccine-preventable and respiratory disease surveillance and coordination (ELC “Project O”). The project focuses on strengthening vaccine-preventable and respiratory disease case-based surveillance and outbreak control, building on the foundation of established surveillance systems (e.g., NNDSS) to provide broader and more representative data.
The ELC “Project O” CoAg has four required activities:
- Coordination of NNDSS surveillance for selected vaccine-preventable and respiratory diseases, including the identification of a VPD Surveillance Coordinator.
- Enhancement of surveillance for meningococcal disease.
- Enhancement of surveillance for varicella.
- Establishment/enhancement of surveillance for acute flaccid myelitis.
Optional activities address varicella, pertussis, Haemophilus influenzae, invasive pneumococcal disease, measles, mumps and other vaccine-preventable and respiratory diseases.
Questions about the ELC “Project O” CoAg or requests for project documents should be directed to VPDsurvELC@cdc.gov.
NNDSS Modernization and the Data Modernization Initiative
NNDSS modernization activities improve CDC surveillance efforts as a component of the Data Modernization Initiative (DMI). DMI is an initiative to modernize core data and surveillance infrastructure across the federal and state public health landscape through building a foundation that supports the goal of connected and sustainable systems (e.g., technology, workforce, and partnerships).
NCIRD has developed a process to support harmonization of data elements for data collection (e.g., using message mapping guides) across the 23 diseases or conditions for which NCIRD provides subject matter expertise. NCIRD considers harmonization to include identification of overlapping and similar concepts across conditions, so that concepts are standardized and harmonized, or a decision is made to retain the concept in a unique format to meet a specific public health requirement.
OMB-PRA approval has been received for the data included in NNDSS. For details, see Federal Register OMB Package 0920-0728.
Worksheets for Disease/Condition Surveillance
Disease surveillance worksheets have been developed as standard tools to assist jurisdictions with case/outbreak reporting, investigation, and notification. These worksheets are intended as a resource to aid with collection of the surveillance data associated with the HL7 message mapping guides (MMGs). Jurisdictions may modify these worksheets as needed, to best support surveillance needs. The worksheets are not required.
The worksheets listed below are presented both with and without annotations; the annotations show applicable HL7 MMG data element (DE) identifiers for easier mapping into surveillance systems. In addition, the most recent versions (e.g., 2019 for measles) of the worksheets incorporate the additional data elements that are included in the HL7 MMGs, both with and without annotations.
Worksheets may be used and modified to meet specific needs, adding and omitting data elements as might be most helpful within the jurisdiction. [Note that the legionella document is a form and therefore may not be modified without OMB approval.] The MMGs, however, are the definitive source for documentation for data elements, valid values, data structure, and other technical issues related to NNDSS data collection.
Vaccine Preventable Diseases
See Vaccine Preventable Diseases web page
Congenital Rubella Syndrome (CRS)
- CRS Worksheet – 2009 version [2 pages]
- CRS Worksheet – 2009 version annotated [2 pages]
- CRS Worksheet – 2019 version [6 pages]
- CRS Worksheet – 2019 version annotated [6 pages]
COVID-19
- View COVID-19 archived surveillance worksheet [5 pages]
- View COVID-19 archived annotated surveillance worksheet [5 pages]
Diphtheria
Haemophilus influenzae
- Hflu Worksheet – Abbreviated Option 2010 [1 page]
- Hflu Worksheet – Abbreviated Option 2010 annotated [1 page]
- Hflu Worksheet – Expanded Option 2010 [3 pages]
- Hflu Worksheet – Expanded Option 2010 annotated [3 pages]
- Hflu Worksheet – 2019 version [5 pages]
- Hflu Worksheet – 2019 version annotated [5 pages]
Measles
- Measles Worksheet – 2009 version [2 pages]
- Measles Worksheet – 2009 version annotated [2 pages]
- Measles Worksheet – 2019 version [4 pages]
- Measles Worksheet – 2019 version annotated [4 pages]
Meningococcal Disease (more coming soon)
- Enhanced Meningococcal Worksheet – 2019 version [2 pages]
- Enhanced Meningococcal Disease Worksheet – 2019 version annotated [2 pages]
- Meningococcal Disease Worksheet – 2021 version [5 pages]
- Meningococcal Disease Worksheet – 2021 version annotated [5 pages]
Mumps
- Mumps Worksheet – 2009 version [2 pages]
- Mumps Worksheet – 2009 version annotated [2 pages]
- Mumps Worksheet – 2021 version [4 pages]
- Mumps Worksheet – 2021 version annotated [4 pages]
Pertussis
- Pertussis Worksheet – 2009 version [2 pages]
- Pertussis Worksheet – 2009 version annotated [3 pages]
- Pertussis Worksheet – 2019 version [5 pages]
- Pertussis Worksheet – 2019 version annotated [5 pages]
Poliomyelitis (paralytic and nonparalytic)
- Suspected Polio Case Worksheet – 2010 version [3 pages]
- Suspected Polio Case Worksheet – 2010 version annotated [3 pages]
Rubella
- Rubella Worksheet – 2009 version [2 pages]
- Rubella Worksheet – 2009 version annotated [2 pages]
- Rubella Worksheet – version 2019 [6 pages]
- Rubella Worksheet – version 2019 annotated [5 pages]
Streptococcus pneumoniae invasive disease
- Invasive Pneumococcal Disease Worksheet – 2010 version [2 pages]
- Invasive Pneumococcal Disease Worksheet – 2010 version annotated [2 pages]
- Invasive Pneumococcal Disease Worksheet – 2019 version [4 pages]
- Invasive Pneumococcal Disease Worksheet – 2019 version annotated [4 pages]
Tetanus
Varicella
- Varicella Worksheet – 2005 version [3 pages]
- Varicella Worksheet – 2005 version annotated [3 pages]
- Varicella Worksheet – 2018 version [4 pages]
- Varicella Worksheet – 2018 version annotated [4 pages]
Non-Vaccine Preventable Diseases
Legionellosis
- Legionellosis CRF – 2020 version [2 pages]*
- Legionellosis CRF – 2020 version annotated [2 pages]
- Legionella Worksheet – 2020 version [5 pages]
- Legionella Worksheet – 2020 version annotated [5 pages]
Psittacosis
- Psittacosis Human Case Report – 2017 version [4 pages]
- Psittacosis Human Case Report Worksheet – 2017 version annotated [4 pages]
- Psittacosis Worksheet – 2020 version [4 pages]
- Psittacosis Worksheet – 2020 version annotated [4 pages]
*The legionella document is a form and therefore may not be modified without OMB approval.
Related Links and Resources:
- Surveillance Trainings & Videos
- Guidance for the Evaluation and Public Health Management of Suspected Outbreak of Meningococcal Disease [4 MB, 16 pages]
- Pan American Health Organization (PAHO) – Immunization
- CSTE – Surveillance/Informatics Steering Committee
- CSTE – VPD Subcommittee
- Mumps [Mumps | Home | CDC]